Abbreviations
APC
cLN
Foxp3+
GC
LN
M3R
RA
SAN
SLE
SS
SSA
SSB
Tfh
Tfr
WT
INTRODUCTION
MATERIALS AND METHODS
Mice
Saliva measurement
Histopathologic examination
FACS analysis
ELISA
ELISPOT assay
RESULTS AND DISCUSSION
SAN mice exhibit manifestations of sialadenitis after the onset of lupus nephritis
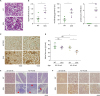 | Figure 1Clinical and histopathologic manifestations akin to lupus nephritis and SS in SAN mice. Female SAN mice and their WT littermates at 40–45 wk (A, B), 20 wk (C), and the indicated wk (D-F) of age were examined by clinical and histopathologic methods. (A) Kidney sections stained with periodic acid-Schiff. (B) Histopathologic indexes. (C) Immunohistochemical images of kidney sections stained with anti-mouse IgG mAb. (D) Saliva flow rates. (E) Salivary gland sections stained with H&E. Arrows indicate foci of lymphoid infiltrates. (F) Immunohistochemical images of salivary gland sections stained with anti-mouse IgG mAb. Photographs are representative of more than three individuals. Graphs display means±SEMs with symbols representing the values of individual mice.HETERO, heterozygote; NS, not significant.
*The p<0.05 by Student's t-test.
|
The production of autoantibodies targeting salivary glands is enhanced in SAN mice from the preclinical phase
 | Figure. 2The spectrum of autoantibodies and frequencies of autoantibody-secreting cells in SAN mice. (A, B) Sera were collected from mice at 40–45 wk of age (A) and at the indicated ages (B), and assayed by ELISA. (C) ASCs in spleen and cervical LNs from 30–35-wk-old mice were counted in ELISPOT assays. Each symbol represents individual mice and horizontal lines display mean±SEM of each group.AU, arbitrary units; ASC, Ab-secreting cell; NS, not significant; SGE, salivary gland extracts; Sp, spleen.
*The p<0.05, **p<0.01 and ***p<0.001 by Student's t-test.
|
The salivary gland-draining LNs in SAN mice contain elevated numbers of Tfh cells and their downstream effector cells
 | Figure. 3FACS profiles of humoral immune cells associated with GC in SAN mice. Humoral immune cells in SAN mice and their WT littermates at 30–35 wk of age were assayed post mortem by FACS. Representative FACS profiles gated on CD4+CD44high cells (A), B220+ cells (B), viable lymphocytes (C) and CD4+ cells (D) are shown in the left panels. Graphs displaying percentages or cell numbers are presented in the right panels. All data are representative of more than 3 independent experiments.NS, not significant; Sp, spleen.
*The p<0.05, **p<0.01, and ***p<0.001 by Student's t-test.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download