Abstract
Objective
To investigate the significance of lung nodule in hydatidiform mole, we retrospectively compared the clinical outcomes of those patients treated with different strategies.
Methods
The patients were divided into three groups: chemotherapy immediately once lung nodule was detected (group 1, n=17), delayed chemotherapy until human chorionic gonadotrophin (hCG) level met the diagnostic criteria for gestational trophoblastic neoplasia (GTN) (group 2, n=18), and hCG surveillance alone until hCG level was normalized spontaneously (group 3, n=18). The clinical parameters of these patients were collected and analyzed.
Results
Totally 53 (4.0%) patients were included from 1,323 cases with molar pregnancy during past 16 years. Among them, the diameters of lung nodules were 0.3–2.5 cm. Chemotherapy cycles for achieving hCG normalization and the failure rate of first-line chemotherapy in group 1 were significantly increased than that in group 2 (5 vs. 3 cycles, p=0.000, 58.8% vs. 11.1%, p=0.005). The hCG level of all 18 cases in group 3 was normalized spontaneously within 6 months. Of those, lung nodules of 9 patients disappeared spontaneously, accounting for 25% (9/36) of patients who initially selected observation. The proportion of single nodule in group 3 was significantly higher than that in group 2 (10/18 vs. 2/18, p=0.012).
Conclusion
Our results suggest that lung nodule alone is not an adequate indication of chemotherapy in molar pregnancy. hCG surveillance is safe for patients with lung nodule, especially with single nodule, as long as their hCG levels do not meet International Federation of Gynecology and Obstetrics diagnostic criteria for GTN.
References
1. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed.Lyon: International Agency for Research on Cancer (IARC);2014.
3. Ngan HY, Kohorn EI, Cole LA, Kurman RJ, Kim SJ, Lurain JR, et al. Trophoblastic disease. Int J Gynaecol Obstet. 2012; 119(Suppl 2):S130–6.

4. Rice LW, Berkowitz RS, Lage JM, Goldstein DP, Bernstein MR. Persistent gestational trophoblastic tumor after partial hydatidiform mole. Gynecol Oncol. 1990; 36:358–62.

5. Page NM, Kemp CF, Butlin DJ, Lowry PJ. Placental peptides as markers of gestational disease. Reproduction. 2002; 123:487–95.

6. Darby S, Jolley I, Pennington S, Hancock BW. Does chest CT matter in the staging of GTN? Gynecol Oncol. 2009; 112:155–60.
7. Braga A, Torres B, Burlá M, Maestá I, Sun SY, Lin L, et al. Is chemotherapy necessary for patients with molar pregnancy and human chorionic gonadotropin serum levels raised but falling at 6months after uterine evacuation? Gynecol Oncol. 2016; 143:558–64.
8. Eysbouts YK, Bulten J, Ottevanger PB, Thomas CM, Ten Kate-Booij MJ, van Herwaarden AE, et al. Trends in incidence for gestational trophoblastic disease over the last 20 years in a population-based study. Gynecol Oncol. 2016; 140:70–5.
9. Newlands ES. The management of recurrent and drug-resistant gestational trophoblastic neoplasia (GTN). Best Pract Res Clin Obstet Gynaecol. 2003; 17:905–23.

11. Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C, et al. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi39–50.

12. Bolze PA, Attia J, Massardier J, Seckl MJ, Massuger L, van Trommel N, et al. Formalised consensus of the European Organisation for Treatment of Trophoblastic Diseases on management of gestational trophoblastic diseases. Eur J Cancer. 2015; 51:1725–31.

13. Sebire NJ, Seckl MJ. Gestational trophoblastic disease: current management of hydatidiform mole. BMJ. 2008; 337:a1193.

14. Kohorn EI. Negotiating a staging and risk factor scoring system for gestational trophoblastic neoplasia. A progress report. J Reprod Med. 2002; 47:445–50.
15. Niimi K, Yamamoto E, Nishino K, Fujiwara S, Ino K, Kikkawa F. Spontaneous regression of gestational trophoblastic neoplasia. Gynecol Oncol Rep. 2017; 21:98–100.

16. Tanaka T, Yanagida S, Yanaihara N. The general rules for clinical and pathological management of trophoblastic diseases–2011, the 3rd edition. Nihon Rinsho. 2012; 70(Suppl 4):695–8.
17. Ring AM. The concept of benign metastasizing hydatidiform moles. Am J Clin Pathol. 1972; 58:111–7.

18. Roberts KE, Hamele-Bena D, Saqi A, Stein CA, Cole RP. Pulmonary tumor embolism: a review of the literature. Am J Med. 2003; 115:228–32.

19. Hertz R. Spontaneous regression in choriocarcinoma and related gestational trophobalstic neoplasms. Natl Cancer Inst Monogr. 1976; 44:59–60.
20. Shaaban AM, Rezvani M, Haroun RR, Kennedy AM, Elsayes KM, Olpin JD, et al. Gestational trophoblastic disease: clinical and imaging features. Radiographics. 2017; 37:681–700.

21. Hanamiya M, Aoki T, Yamashita Y, Kawanami S, Korogi Y. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol. 2012; 81:152–7.

22. Yamashita Y, Torashima M, Takahashi M, Mizutani H, Miyazaki K, Matsuura K, et al. Contrast-enhanced dynamic MR imaging of postmolar gestational trophoblastic disease. Acta Radiol. 1995; 36:188–92.

23. Tidy JA, Hancock B, Ansink A, Hammond CB, Ilancheran A, Jauniaux ER, et al. The management of gestational trophoblastic neoplasia. Royal College of Obstetricians and Gynaecologists, guideline No. 38. London: RCOG Press;2010.
24. Gerulath AH, Ehlen TG, Bessette P, Jolicoeur L, Savoie R, et al. Society of Obstetricians and Gynaecologists of Canada Gestational trophoblastic disease. J Obstet Gynaecol Can. 2002; 24:434–46.
25. Soper JT, Mutch DG, Schink JC. American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol. 2004; 93:575–85.
Fig. 1.
The chemotherapy response for patients in group 1 and 2. (A) More chemotherapy cycles for achieving human chorionic gonadotrophin normalization were used in group 1 than that in group 2. (B) More failure rate of first-line chemotherapy in group 1 than that in group 2.
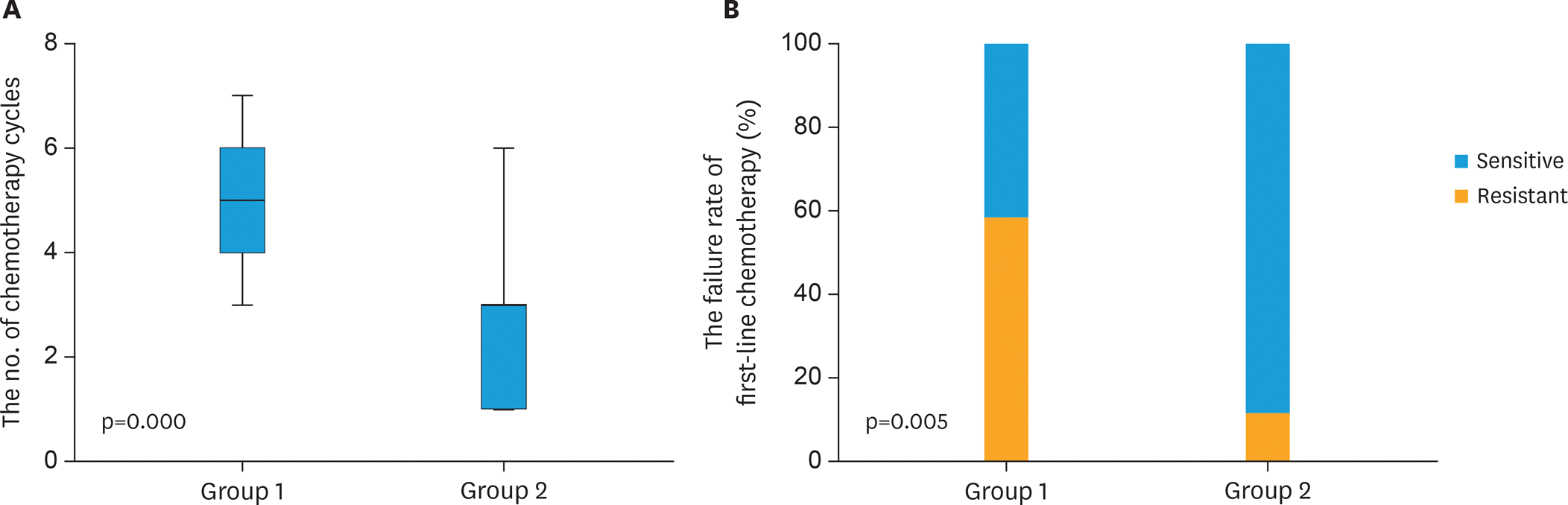
Table 1.
The clinical characteristics of patients in three groups
| Variables | Group 1 (n=17) | Group 2 (n=18) | Group 3 (n=18) | p-value |
|---|---|---|---|---|
| Age (yr) | 26 (17–53) | 29.5 (17–53) | 31.5 (16–54) | 0.369 |
| hCG level of pre-evacuation (IU/L) | 464,672.0 (50,138.0–2,004,065.0) | 152,386.0 (1,000.0–1,000,000.0) | 140,880.5 (14,812.9–431,000.0) | 0.107 |
| Gestational age (day) | 74 (46–147) | 71 (37–270) | 69 (30–112) | 0.111 |
| Pathology of molar pregnancy | 0.231 | |||
| CHM | 17 | 16 | 15 | |
| PHM | 0 | 2 | 3 | |
| Detected time of lung nodule* | 0.281 | |||
| At evacuation | 12 | 11 | 8 | |
| After evacuation | 5 | 7 | 10 | |
| The largest diameter of lung nodule (cm) | 0.6 (0.3–1.7) | 0.6 (0.3–2.5) | 0.65 (0.3–1.7) | 0.946 |
| The quantity of lung nodule | 0.018† | |||
| Single | 7 | 2 | 10 | |
| Multiple | 10 | 16 | 8 |
Table 2.
The clinical characteristics related with chemotherapy in group 1 and 2
Table 3.
The response to chemotherapy for patients in group 1 and 2 by logistic regression analysis




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download