1. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999; 341:625–634. PMID:
10460813.
2. Sorajja P, Borlaug BA, Dimas VV, et al. SCAI/HFSA clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. Catheter Cardiovasc Interv. 2017; 89:E233–E247. PMID:
28489331.
3. van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017; 136:e232–e268. PMID:
28923988.
4. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017; 377:2419–2432. PMID:
29083953.
5. Truong HT, Hunter G, Lotun K, et al. Insertion of the Impella via the axillary artery for high-risk percutaneous coronary intervention. Cardiovasc Revasc Med. 2018; 19:540–544. PMID:
29422276.
6. Mathur M, Hira RS, Smith BM, Lombardi WL, McCabe JM. Fully percutaneous technique for transaxillary implantation of the Impella CP. JACC Cardiovasc Interv. 2016; 9:1196–1198. PMID:
27282605.
7. Kamioka N, Patel A, Burke MA, Greenbaum A, Babaliaros V. Biventricular Impella placement via complete venous access. Catheter Cardiovasc Interv. 2017.
8. Kantrowitz A, Tjønneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968; 203:113–118. PMID:
5694059.
9. De Silva K, Lumley M, Kailey B, et al. Coronary and microvascular physiology during intra-aortic balloon counterpulsation. JACC Cardiovasc Interv. 2014; 7:631–640. PMID:
24726295.
10. Ouweneel M, Eriksen E, Sjauw KD, et al. Impella CP versus intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock: the IMPRESS trial. J Am Coll Cardiol. 2016.
11. Briceno N, Ryan M, O'Gallagher K, et al. TCT-450 Coronary and myocardial haemodynamic effects of Impella LV unloading in patients undergoing high-risk percutaneous coronary intervention. J Am Coll Cardiol. 2018; 72:B181.
12. Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008; 52:1584–1588. PMID:
19007597.
13. Valgimigli M, Steendijk P, Sianos G, Onderwater E, Serruys PW. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2.5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005; 65:263–267. PMID:
15895406.
14. Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007; 70:532–537. PMID:
17896398.
15. Moriyama N, Lindström L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA versus ProGlide. EuroIntervention. 2018; pii: EIJ-D-18-00769.
16. Kapur NK, Lala A, Josephy N, Guo Y, Karas RH, Burkhoff D. TCT-196 the recover right trial criteria for right ventricular failure: an analysis of the SHould we emergently revascularize Occluded coronaries for Cardiogenic shock (SHOCK) Trial and Registry. J Am Coll Cardiol. 2015; 66:B74–B75.
17. Kuchibhotla S, Esposito ML, Breton C, et al. Acute biventricular mechanical circulatory support for cardiogenic shock. J Am Heart Assoc. 2017; 6:e006670. PMID:
29054842.
18. Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015; 34:1549–1560. PMID:
26681124.
19. Anderson M, Morris DL, Tang D, et al. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J Heart Lung Transplant. 2018; 37:1448–1458. PMID:
30241890.
20. Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008; 17(Suppl 4):S41–S47. PMID:
18964254.
21. Schrage B, Burkhoff D, Rübsamen N, et al. Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. JACC Heart Fail. 2018; 6:1035–1043. PMID:
30497643.
22. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016; 44:1974–1979. PMID:
27322361.
23. Baruteau AE, Barnetche T, Morin L, et al. Percutaneous balloon atrial septostomy on top of venoarterial extracorporeal membrane oxygenation results in safe and effective left heart decompression. Eur Heart J Acute Cardiovasc Care. 2018; 7:70–79. PMID:
27742755.
24. Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009; 30:459–468. PMID:
19168529.
25. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287–1296. PMID:
22920912.
26. Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013; 382:1638–1645. PMID:
24011548.
27. Thiele H, Zeymer U, Thelemann N, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2018.
28. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:e240–e327. PMID:
23741058.
29. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39:119–177. PMID:
28886621.
30. Hawranek M, Gierlotka M, Pres D, Zembala M, Gąsior M. Nonroutine use of intra-aortic balloon pump in cardiogenic shock complicating myocardial infarction with successful and unsuccessful primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2018; 11:1885–1893. PMID:
30236362.
31. van Nunen LX, van't Veer M, Schampaert S, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction: old and emerging indications. Neth Heart J. 2013; 21:554–560. PMID:
24170231.
32. O'Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018; 202:33–38. PMID:
29803984.
33. Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017; 69:278–287. PMID:
27810347.
35. Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015; 36:2246–2256. PMID:
26033984.
37. Lima B, Kale P, Gonzalez-Stawinski GV, Kuiper JJ, Carey S, Hall SA. Effectiveness and safety of the Impella 5.0 as a bridge to cardiac transplantation or durable left ventricular assist device. Am J Cardiol. 2016; 117:1622–1628. PMID:
27061705.
38. Hall SA, Carey S, Edens M, et al. Use of Impella 5.0 ventricular assist device as a bridge to decision during acute decompensation of end-stage chronic heart failure. J Heart Lung Transplant. 2016; 35:S54–S55.
39. Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018; 91:454–461. PMID:
29266676.
40. Perera D, Stables R, Clayton T, et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013; 127:207–212. PMID:
23224207.
41. Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010; 304:867–874. PMID:
20736470.
42. O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012; 126:1717–1727. PMID:
22935569.
43. Henriques JP, Ouweneel DM, Naidu SS, et al. Evaluating the learning curve in the prospective Randomized Clinical Trial of hemodynamic support with Impella 2.5 versus Intra-Aortic Balloon Pump in patients undergoing high-risk percutaneous coronary intervention: a prespecified subanalysis of the PROTECT II study. Am Heart J. 2014; 167:472–479.e5. PMID:
24655695.
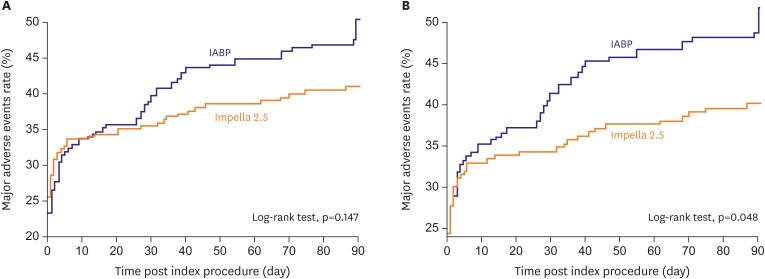




 PDF
PDF ePub
ePub Citation
Citation Print
Print



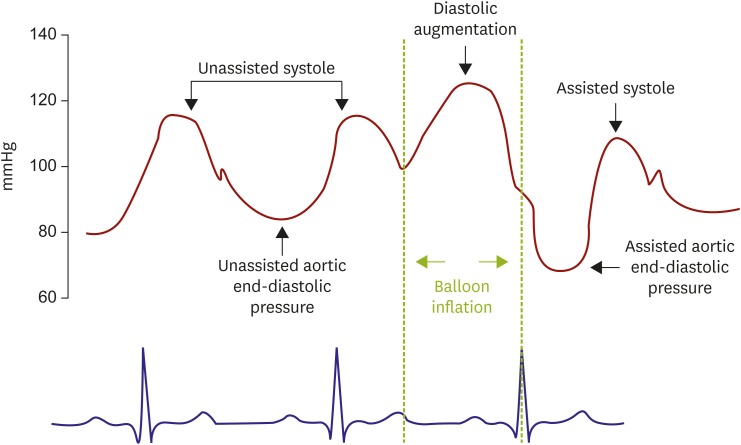
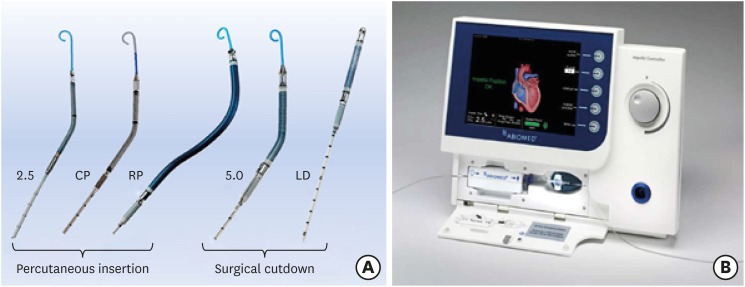
 XML Download
XML Download