INTRODUCTION
Hepatic artery occlusion (HAO) after liver transplantation (LT) is a devastating complication (
12). HAO is a wide-spectrum entity, ranging from partial HAO (i.e., significant partial stenosis or partial thrombosis) to near-complete or complete HAO (i.e., complete occlusion by stenosis or thrombosis). Significant hepatic artery (HA) stenosis and thrombosis may coexist and be synergistic. HAO can progress to acute bile duct necrosis with or without biliary sepsis, early graft failure, or even mortality (
1345). Therefore, early detection of HAO and its timely management are crucial for a favorable outcome in LT recipients (
67).
Doppler ultrasound (US) is an established surveillance method for HAO in LT recipients. However, previous studies have shown that, although Doppler US is sensitive for diagnosing HAO, it has a low positive-predictive value (PPV) and a high false-positive rate (
89). This may occur particularly when a small HA is close to a large portal vein and the weak signal from the HA is masked by the blooming signal from the portal vein (
8). When HAO is suspected on Doppler US, hepatic arteriography is commonly indicated to confirm diagnosis. However, considering the invasiveness and potential complications of hepatic arteriography, the use of an appropriate second-line imaging tool that could complement the low specificity of Doppler US would be adequate, before resorting to arteriography.
Contrast-enhanced ultrasound (CEUS) provides real-time angiographic-like images using a microbubble contrast agent. The efficacy of CEUS as a non-invasive technique for diagnosing HAO after LT has been validated by several investigators (
1011). Previous studies have usually used non-visualization of HA on CEUS in patients with no Doppler detectable HA flow as the criterion for diagnosing HAO. When this criterion is used in patients with no flow on Doppler US, with near-complete or complete HAO, CEUS showed almost perfect sensitivity and specificity (
912). However, in patients with partial HAO, presenting with tardus-parvus HA pattern on Doppler US, a previous study suggested that CEUS had only a limited role (
13). Therefore, it is helpful to identify other imaging findings on CEUS that could improve over the conventional criterion (i.e., no visible HA) to achieve an exact diagnosis of HAO and to reduce or even eliminate the need for invasive angiography.
The purpose of our study was to investigate whether the diagnostic performance of CEUS could be improved by modifying the conventional criterion to diagnose HAO after LT, and furthermore, to establish the role of CEUS in patients with a tardus-parvus HA pattern on Doppler US, using modification of the conventional criterion.
Go to :

MATERIALS AND METHODS
The relevant Institutional Review Board approved this study and the need to obtain informed patient consent was waived due to the retrospective nature of the analyses.
Subjects
Between January 2010 and February 2017, 2679 adult LTs (18 years or older) were performed at a single institution. Among them, 288 recipients (10.8%) were suspected of having HAO by Doppler US during hospitalization. The Doppler US criteria for HAO included no Doppler signal or a tardus-parvus waveform (with resistive index < 0.5 and systolic acceleration time > 0.08 seconds) at the graft HA or a focal high velocity jet > 2 m/s at the anastomosis (
1415). The institution where this study was conducted has an extensive radiologic postoperative complication surveillance program: routine Doppler USs are performed daily during the first week after surgery and thereafter once or twice per week during the hospitalization period. Additional studies are performed at any time when there are clinical indications (i.e., elevation of liver enzyme). We excluded 158 patients for whom CEUS was not obtained within 24 hours after viewing a Doppler abnormality. Two patients were excluded due to technical failure of CEUS.
Finally, 128 recipients with a mean age of 52.0 years ± 10.3 (82 male [52.0 years ± 9.9; range, 25–74 years] and 46 female [52.5 years ± 11.1; range, 21–68 years]) were included. If the patients had undergone multiple CEUS examinations after a Doppler abnormality was found and before discharge, the patient's earliest examination was selected for analysis.
Figure 1 is a flow diagram of the study population. Based on the abnormality pattern on Doppler US, we categorized patients into two subgroups: group A (no flow, n = 57) and group B (tardus-parvus waveform, n = 71). No patient was diagnosed as HAO with a focal high velocity jet > 2 m/s at or around the anastomosis. We reviewed electronic medical records to obtain the liver enzyme levels (i.e., aspartate transaminase, AST, alanine aminotransferase, ALT) on the day before and the day of Doppler abnormality detection.
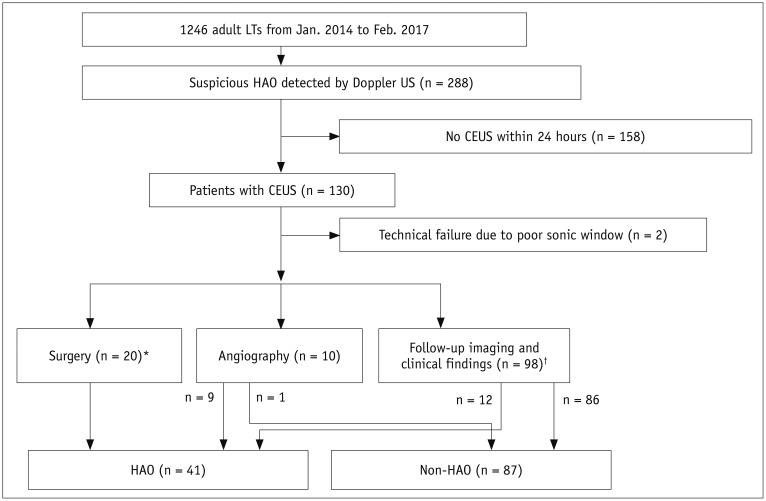 | Fig. 1
Flow diagram describing categories of this study population.
*Surgery included angioplasty (n = 12) and liver retransplantation (n = 8), †Follow-up imaging and clinical findings means that we determined HAO as persistent no flow or progressive change from tardus-parvus pattern to no flow on Doppler US follow-up studies, which was associated with development of multifocal subsegmental ischemia or infarction or bile duct necrosis or biloma due to HAO. We defined non-HAO as normalization of Doppler US abnormalities, without graft ischemia or infarction within 6 months of follow-up. CEUS = contrast-enhanced ultrasound, HAO = hepatic artery occlusion, LT = liver transplantation, US = ultrasound

|
CEUS Methods
CEUS was performed by board-certified abdominal radiology fellows (< 2 years' experience of LT imaging) under the supervision of a staff radiologist (more than 10 years' experience) after administration of SonoVue™ (Bracco Imaging, Milan, Italy). Scans were performed using a Sequoia 512 scanner (Acuson Siemens, Mountain View, CA, USA) with a 1–4-MHz transducer, in 106 patients, or a Toshiba Aplio 500 (Canon Medical Systems Corporation, Tokyo, Japan) with a 1.6–6.0-MHz transducer, in 22 patients. After manually mixing a bottle of SonoVue with 5 mL saline, one-half of the mixture was administered intravenously as a manual bolus injection into the central or peripheral line, at a rate of 1 mL/s, followed by 5 mL of normal saline for flushing. The graft HA was evaluated using a contrast pulse sequencing (contrast-coherent imaging) or contrast harmonic imaging mode. CEUS imaging was recorded at intervals of 1 second or less from injection of contrast and until 100 seconds after all parenchyma were enhanced.
Interpretation of Images
CEUS images were anonymized, coded, and saved in a picture archiving and communication system folder. Two board-certified radiologists (reviewer 1 with > 10 years' experience and reviewer 2 with 3 years' experience), who were blinded to the final outcome and to each other's results, evaluated all CEUS examinations independently for evaluation of the degree of agreement. A consensus review was performed by the two reviewers after completing the independent review sessions, in order to resolve any discrepancies between the two readers.
We evaluated two phases: the hepatic arterial phase and the portal-parenchymal phase. The hepatic arterial phase was qualitatively defined as the time from contrast agent arriving in the graft HA to partial opacification of the main portal vein (half the area of the vein at visual assessment; this usually started within 10–20 seconds after injection and continued to 30–45 seconds). The portal-venous phase was qualitatively defined as the time from complete opacification of the main portal vein to peak enhancement of the hepatic parenchyma (this usually began at about 30–45 seconds and lasts until 2 minutes after injection) (
161718).
We defined the conventional criterion of HAO as no HA enhancement. For evaluation of other findings potentially helpful for HAO diagnosis, abnormal HA enhancement (i.e., delayed, faint, and discontinuous enhancement of HA) and perfusion defect of the liver parenchyma in the portal-venous phase were investigated. Delayed enhancement of HA was determined as enhancement of the HA in the portal-parenchymal phase and faint enhancement of HA was determined as weaker enhancement of HA than that of the portal vein (
Fig. 2). Discontinuous enhancement was determined when some parts of the HA were invisible (
Fig. 3). A perfusion defect of the liver parenchyma was determined as a lack of enhancement in a wedge-shaped, rounded or oval, or irregularly shaped area in the peripheral or central area of the graft. It excluded a flat, decreased enhancement area in the peripheral portion of deceased-donor liver transplants (i.e., cold ischemia) and a wedge-shaped area according to the venous territory with hyperenhancement on the arterial phase and hypoenhancement in the portal-venous phase in living-donor liver transplants (venous congestion).
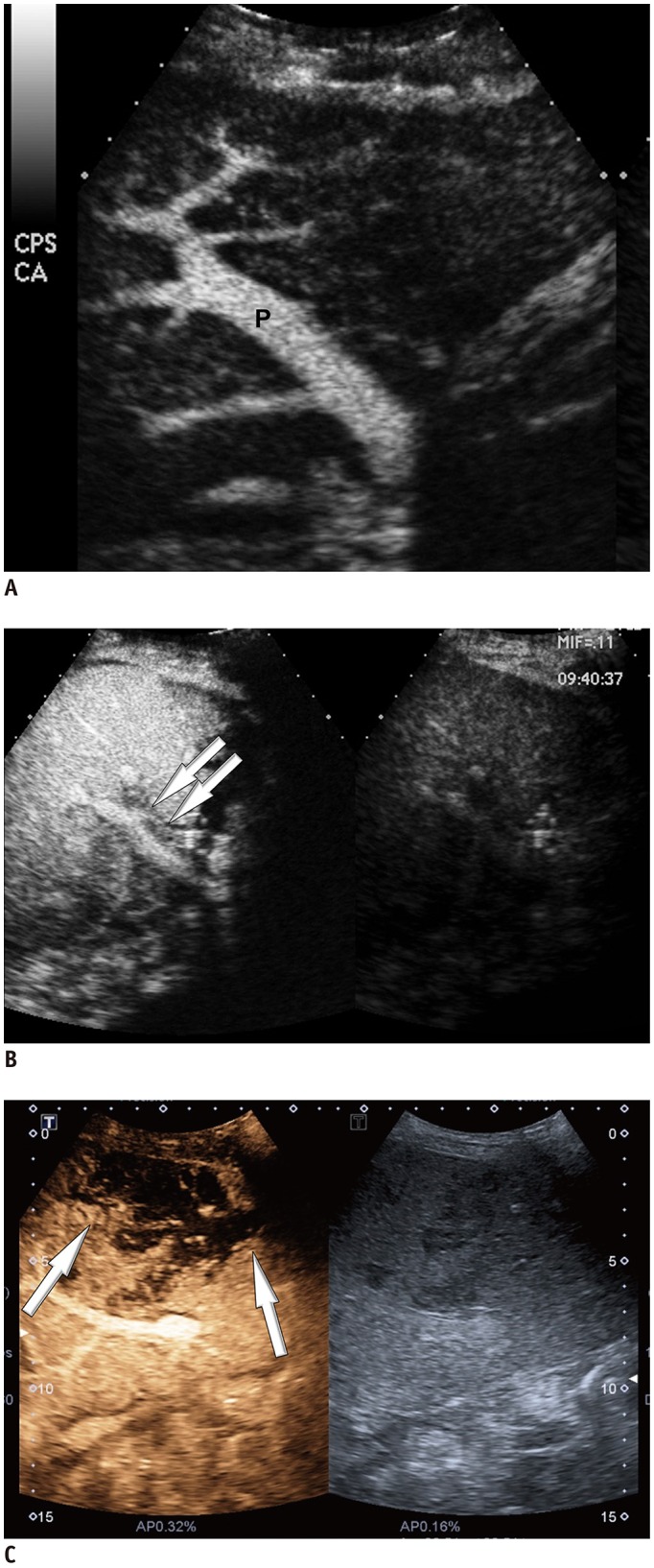 | Fig. 2
CEUS images matching abnormality.
A. CEUS image shows no visible intra-HA flow around portal vein (P). B. CEUS image shows faint and discontinuous enhancement of HA on portal-parenchymal phase (arrows). C. CEUS image shows perfusion defect of liver parenchyma (arrows). HA = hepatic artery

|
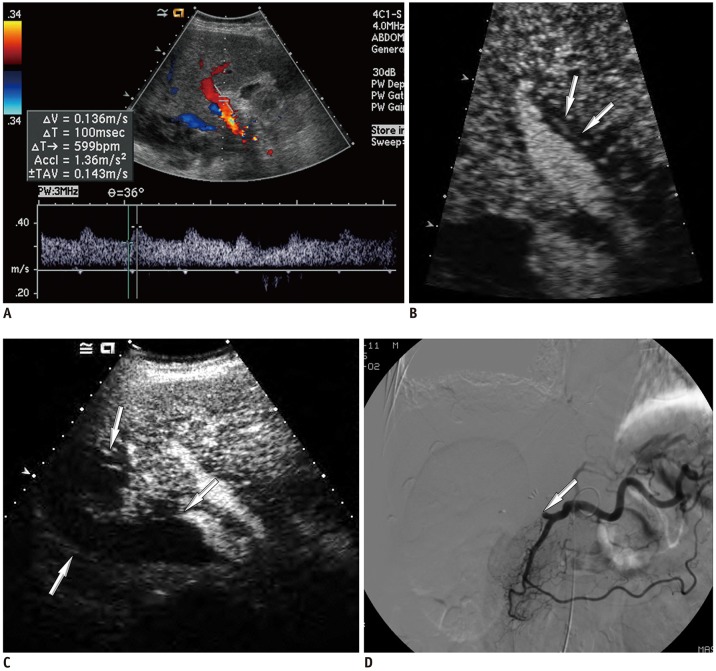 | Fig. 3
False-negative diagnosis based on conventional criterion (no HA enhancement) of CEUS in 54-year-old male, after deceased-donor LT, with tardus-parvus pattern on Doppler US.
A. Doppler US image shows tardus-parvus pattern on Doppler US. B. CEUS image shows faint and discontinuous enhancement of HA in portal-parenchymal phase (arrows). C. CEUS image shows perfusion defect of liver parenchyma (arrows). D. Angiography shows no visible intra-HA flow after proper HA (arrow).

|
Clinical Outcomes
To determine the clinical outcome, a board-certified radiologist (with 3 years' experience) and a LT surgeon (with 3 years' experience) reviewed the radiological and medical records of the patients, categorizing them into the presence or absence of HAO (
Fig. 1). We defined HAO as a totally occluded HA or significant stenosis causing complications. A reference diagnosis of HAO was made by surgery (HA revision or retransplantation due to graft failure related to HAO), hepatic arteriography (from near-total or total occlusion to luminal diameter < 50% and flow disturbance), or follow-up imaging and clinical findings, as follows: persistent no-flow or progressive change from a tardus-parvus pattern to no-flow on Doppler US follow-up studies associated with development of multifocal subsegmental ischemia/infarction or non-anastomotic biliary complications, such as bile duct necrosis/biloma on follow-up CT or CEUS, and a consistent clinical finding of graft dysfunction or even failure. We defined non-HAO as normalization of Doppler US abnormalities and a lack of the above described complications within 6 months of follow-up.
Statistical Analysis
All statistical analyses were performed using commercially available statistical software SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). A p value of < 0.05 was considered statistically significant.
The demographics, findings, and parametric data derived from CEUS of the two groups (HAO vs. non-HAO) were compared using Student's t test for continuous variables, after testing for normality using a Kolmogorov-Smirnov test, and chi-square test for discrete variables in the patients overall, group A (patients with no flow on Doppler US), and group B (patients with tardus-parvus waveform on Doppler US). Additionally, binary logistic regression analysis for selection of significant variables was performed to determine independent parameters for HAO from among the parameters that showed statistical significance in univariate analysis. The sensitivity, specificity, PPV, negative-predictive value (NPV), and accuracy of statistically significant CEUS findings in the diagnosis of HAO were calculated. To find the optimal modified criteria for CEUS with best performance for diagnosing HAO, the areas under the receiver operating characteristic curves (AUCs) of CEUS abnormalities were compared.
To assess interobserver variability, kappa statistics were used. A κ value of 0.21–0.40 indicated poor agreement; 0.41–0.60, fair agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement (
19).
Go to :

RESULTS
Figure 1 illustrates the distribution of the study population in each category. Among the 128 patients, 41 patients had true HAO. Recipients with HAO had a first documented abnormality on Doppler US at a mean of 24.2 days ± 59.6 (range, 0–359 days; median, 11 day; interquartile range, 2–19 days) after LT. HAO was confirmed by surgery (HA revision [n = 12] or re-transplantation due to graft failure related to HAO [n = 8]), angiography (n = 9), and follow-up studies (n = 12). Twelve patients suffered mortality due to graft failure (29.3%). The mean duration between the CEUS and angiography or HA revision was 1.0 days ± 1.2 (range, 0–3 days).
Table 1 shows the recipient characteristics in each category (HAO and non-HAO groups). HAO occurred more frequently in females than in males (
p = 0.004) and in patients with no detectable flow than in patients with a tardus-parvus wave form on Doppler US (
p < 0.001). There were no significant differences in liver enzymes on the day before or the day of Doppler abnormalities or their ratios between the HAO and non-HAO groups in the patients overall (
Table 1), group A, and group B: group A, HAO group vs. non-HAO group, AST on the day before Doppler abnormalities, 275.5 ± 591.4 vs. 93.3 ± 76.9 (
p = 0.089); AST on the day of Doppler abnormalities, 399.8 ± 663.7 vs. 277.9 ± 625.5 (
p = 0.492); AST ratio, 2.2 ± 2.6 vs. 3.4 ± 5.1 (
p = 0.335); ALT on the day before Doppler abnormalities, 246.9 ± 460.3 vs. 115.0 ± 148.8 (
p = 0.191); ALT on the day of Doppler abnormalities, 365.1 ± 489.5 vs. 201.0 ± 252.3 (
p = 0.107); ALT ratio, 2.6 ± 3.1 vs. 4.5 ± 7.4 (
p = 0.262); group B, HAO group vs. non-HAO group, AST on the day before Doppler abnormalities, 1104.0 ± 2708.6 vs. 441.2 ± 1143.8 (
p = 0.544); AST on the day of Doppler abnormalities, 1172.9 ± 2034.8 vs. 509.3 ± 972.0 (
p = 0.425); AST ratio, 10.8 ± 20.4 vs. 2.2 ± 3.0 (
p = 0.307); ALT on the day before Doppler abnormalities, 522.4 ± 1164.3 vs. 279.0 ± 432.7 (
p = 0.602); ALT on the day of Doppler abnormalities, 515.3 ± 460.1 vs. 333.4 ± 500.1 (
p = 0.361); ALT ratio, 15.2 ± 34.0 vs. 2.4 ± 4.4 (
p = 0.360).
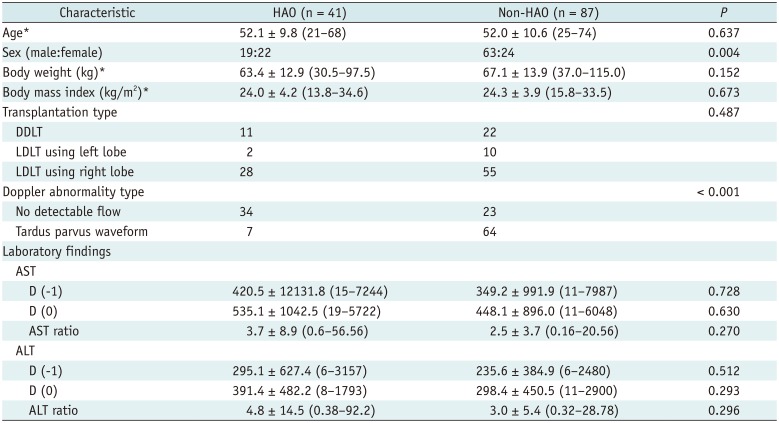
Table 1
Recipient Characteristics in HAO and Non-HAO Groups after Liver Transplantation
|
Characteristic |
HAO (n = 41) |
Non-HAO (n = 87) |
P
|
|
Age*
|
52.1 ± 9.8 (21–68) |
52.0 ± 10.6 (25–74) |
0.637 |
|
Sex (male:female) |
19:22 |
63:24 |
0.004 |
|
Body weight (kg)*
|
63.4 ± 12.9 (30.5–97.5) |
67.1 ± 13.9 (37.0–115.0) |
0.152 |
|
Body mass index (kg/m2)*
|
24.0 ± 4.2 (13.8–34.6) |
24.3 ± 3.9 (15.8–33.5) |
0.673 |
|
Transplantation type |
|
|
0.487 |
|
DDLT |
11 |
22 |
|
|
LDLT using left lobe |
2 |
10 |
|
|
LDLT using right lobe |
28 |
55 |
|
|
Doppler abnormality type |
|
|
< 0.001 |
|
No detectable flow |
34 |
23 |
|
|
Tardus parvus waveform |
7 |
64 |
|
|
Laboratory findings |
|
|
|
|
AST |
|
|
|
|
D (-1) |
420.5 ± 12131.8 (15–7244) |
349.2 ± 991.9 (11–7987) |
0.728 |
|
D (0) |
535.1 ± 1042.5 (19–5722) |
448.1 ± 896.0 (11–6048) |
0.630 |
|
AST ratio |
3.7 ± 8.9 (0.6–56.56) |
2.5 ± 3.7 (0.16–20.56) |
0.270 |
|
ALT |
|
|
|
|
D (-1) |
295.1 ± 627.4 (6–3157) |
235.6 ± 384.9 (6–2480) |
0.512 |
|
D (0) |
391.4 ± 482.2 (8–1793) |
298.4 ± 450.5 (11–2900) |
0.293 |
|
ALT ratio |
4.8 ± 14.5 (0.38–92.2) |
3.0 ± 5.4 (0.32–28.78) |
0.296 |

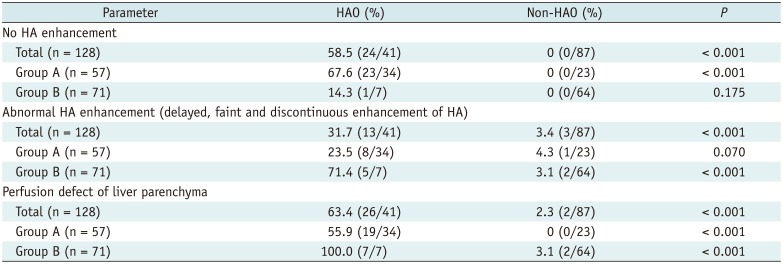
No HA enhancement, abnormal HA enhancement, and perfusion defects of the liver parenchyma were significantly more common in patients with HAO (all, p < 0.001). In binary logistic regression analysis, no HA enhancement (hazard ratios [HR] = 460.80, 95% confidence interval [CI] = 38.83–5467.55, p < 0.001), abnormal HA enhancement (HR = 49.96, 95% CI = 6.70–372.60, p = 0.001), and perfusion defect of liver parenchyma (HR = 54.12, 95% CI = 6.09–480.97, p = 0.0003) were independent predictors for HAO.
In the subgroup analysis with 57 patients with no flow on Doppler US (group A), no HA enhancement and perfusion defect of liver parenchyma were statistically significantly more frequently seen on CEUS (
p < 0.001), whereas abnormal HA enhancement and perfusion defect of liver parenchyma were significantly more common in patients with tardus-parvus pattern on Doppler US (
p < 0.001) (
Table 2).
Table 2
CEUS Findings in HAO and Non-HAO Groups
|
Parameter |
HAO (%) |
Non-HAO (%) |
P
|
|
No HA enhancement |
|
|
|
|
Total (n = 128) |
58.5 (24/41) |
0 (0/87) |
< 0.001 |
|
Group A (n = 57) |
67.6 (23/34) |
0 (0/23) |
< 0.001 |
|
Group B (n = 71) |
14.3 (1/7) |
0 (0/64) |
0.175 |
|
Abnormal HA enhancement (delayed, faint and discontinuous enhancement of HA) |
|
|
|
|
Total (n = 128) |
31.7 (13/41) |
3.4 (3/87) |
< 0.001 |
|
Group A (n = 57) |
23.5 (8/34) |
4.3 (1/23) |
0.070 |
|
Group B (n = 71) |
71.4 (5/7) |
3.1 (2/64) |
< 0.001 |
|
Perfusion defect of liver parenchyma |
|
|
|
|
Total (n = 128) |
63.4 (26/41) |
2.3 (2/87) |
< 0.001 |
|
Group A (n = 57) |
55.9 (19/34) |
0 (0/23) |
< 0.001 |
|
Group B (n = 71) |
100.0 (7/7) |
3.1 (2/64) |
< 0.001 |

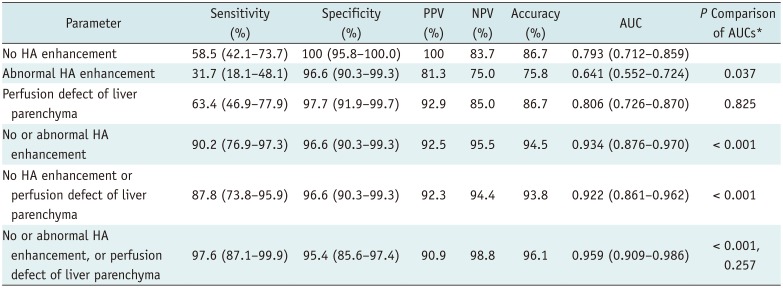
By using the conventional criterion (no HA enhancement alone) to diagnose HAO, the sensitivity, specificity, PPV, NPV, and accuracy were 58.5% (24/41), 100% (87/87), 100% (24/24), and 86.7% (111/128), respectively, in patients overall. The modified criteria of HAO that showed highest AUC were no HA enhancement, abnormal HA enhancement, or perfusion defect of liver parenchyma (
Table 3). Diagnostic performance of modified criteria was significantly better than that of conventional criterion, yielding 97.6% (40/41), 95.4% (83/87), 90.9% (40/44), 98.8% (83/84), and 96.1% (123/128) (
p < 0.001), respectively. In subgroup analysis, diagnostic values of modified criteria in group A (patients with no flow on Doppler US) were as follows: sensitivity, 97.1% (33/34); specificity, 95.7% (22/23); PPV, 97.1% (33/34), NPV, 95.7% (22/23); accuracy, 96.5% (55/57). Those values in group B (patients with tardus-parvus pattern) were as follows: sensitivity, 100% (7/7); specificity, 95.3% (61/64); PPV, 70.0% (7/10), NPV, 100% (61/61); accuracy, 95.8% (68/71) (
Table 4,
Fig. 3). CEUS was performed using Sequoia 512 scanner in 106 patients and using Aplio 500 in 22 patients. There was no significant difference in accuracy between the two US systems (
p = 0.453).
Table 3
Diagnostic Performance of CEUS Abnormalities to Determine Modified Criteria for HAO
|
Parameter |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
AUC |
P Comparison of AUCs*
|
|
No HA enhancement |
58.5 (42.1–73.7) |
100 (95.8–100.0) |
100 |
83.7 |
86.7 |
0.793 (0.712–0.859) |
|
|
Abnormal HA enhancement |
31.7 (18.1–48.1) |
96.6 (90.3–99.3) |
81.3 |
75 |
75.8 |
0.641 (0.552–0.724) |
0.037 |
|
Perfusion defect of liver parenchyma |
63.4 (46.9–77.9) |
97.7 (91.9–99.7) |
92.9 |
85 |
86.7 |
0.806 (0.726–0.870) |
0.825 |
|
No or abnormal HA enhancement |
87.8 (73.8–95.9) |
96.6 (90.3–99.3) |
92.3 |
94.4 |
93.8 |
0.922 (0.861–0.962) |
< 0.001 |
|
No HA enhancement or perfusion defect of liver parenchyma |
90.2 (76.9–97.3) |
96.6 (90.3–99.3) |
92.5 |
95.5 |
94.5 |
0.934 (0.876–0.970) |
< 0.001 |
|
No or abnormal HA enhancement, or perfusion defect of liver parenchyma |
97.6 (87.1–99.9) |
95.4 (85.6–97.4) |
90.9 |
98.8 |
96.1 |
0.959 (0.909–0.986) |
< 0.001, 0.257 |

Table 4
Diagnostic Performance of Modified Criteria for HAO in Subgroups

|
Sub-Group |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
AUC |
|
Group A (n = 57) |
97.1 (84.7–99.9) |
95.7 (78.1–99.9) |
97.1 |
95.7 |
96.5 |
0.96 (0.88–1.00) |
|
Group B (n = 71) |
100.0 (59.0–100.0) |
95.3 (86.9–99.0) |
70.0 |
100.0 |
95.8 |
0.99 (0.91–1.00) |

Interobserver agreements for all criteria of CEUS (no HA enhancement, κ = 1.00; abnormal HA enhancement, κ = 0.929; perfusion defect of liver parenchyma, κ = 1.00) were excellent. Discordance of the assigned category between the two readers was observed only in 2/128 patients (1.6%), using the criterion of abnormal HA enhancement.
Go to :

DISCUSSION
HAO is considered as a serious threat to graft survival after LT. Therefore, early diagnosis and treatment is important for its successful management (
1). CEUS could be used as a second-line approach for evaluating HAO after LT, when abnormality is suspected on Doppler US. With the advantage of being a bedside examination, CEUS has complemented Doppler US in many previous studies, particularly in patients who cannot undergo contrast-enhanced CT because of azotemia or whose vital signs are not stable (
122021).
Previous studies have usually used non-visualization of HA on CEUS as the criterion for HAO and have used complete occlusion of the HA on angiography as the reference standard for HAO (
912). However, it is also important to diagnose significant partial HAO, such as significant stenosis, as this can rapidly progress to near-complete or complete HAO, resulting in severe complications, such as multifocal infarctions, bile duct necrosis-biloma, graft failure, and mortality. Therefore, a new, extended criterion is needed to cover this gray zone (i.e., partial HAO). In our results, modified HAO criteria, which showed the highest AUC, were no HA enhancement, abnormal HA enhancement, or perfusion defect of liver parenchyma, which showed significantly increased sensitivity (97.6%, 40/41), accuracy (96.1%, 123/128), and AUC (0.959), as compared with those of the single conventional criterion (i.e., no HA enhancement) (sensitivity, 58.5% [24/41]; accuracy, 86.7% [111/128]; AUC, 0.793) (
p < 0.001, respectively).
In most previous studies that have focused on the role of CEUS in patients with no Doppler-detectable flow, the role of CEUS in patients with a tardus-parvus HA pattern has been not well addressed (
921). One previous study even suggested that CEUS had little role in patients with a tardus-parvus pattern (
13). In our study, we included a considerable number of patients (n = 71) with a tardus-parvus waveform on Doppler US, although the prevalence of HAO in patients with a tardus-parvus waveform on Doppler US (group B, 9.9% [7/71]) was lower than that in patients with no flow on Doppler US (group A, 59.6% [34/57]). Particularly in group B, the sensitivity of the conventional CEUS criterion for HAO was only 14.3% (1/7). Using the modified criteria, the sensitivity increased in group B (100% [7/7]) as well as in group A (97.1% [33/34]), without significant sacrifice of specificity (group B, 95.3% [61/64]; group A, 95.7% [22/23]).
There were several limitations in the present study. First, there was an inherent limitation due to the retrospective study design, implying that this study was subject to heterogeneity of CEUS methods and interobserver variation in interpretation. However, in our institution, CEUS examinations are strictly controlled by a supervisor, and the protocol for acquisition of semi-real-time data for HA was rather simple. Subsequently, interobserver agreements were excellent. Second, in our study, HAO occurred more frequently in females than in males (p = 0.004) for reasons that are not immediately clear. However, the purpose of our current study was not to elucidate the causes of HAO.
In conclusion, our modified criteria (no HA enhancement, abnormal HA enhancement, or perfusion defect of liver parenchyma) could improve diagnostic performance of CEUS for diagnosing HAO, particularly by increasing sensitivity. By using these modified criteria, CEUS could be useful for diagnosing HAO, even in patients with tardus-parvus pattern on Doppler US.
Go to :












 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download