INTRODUCTION
MATERIALS AND METHODS
Search Strategy and Eligibility Criteria
Data Extraction and Study Endpoints
Quality Assessment
Data Synthesis and Statistical Analysis
RESULTS
Literature Selection
Study Characteristics
Table 1
Study Characteristics
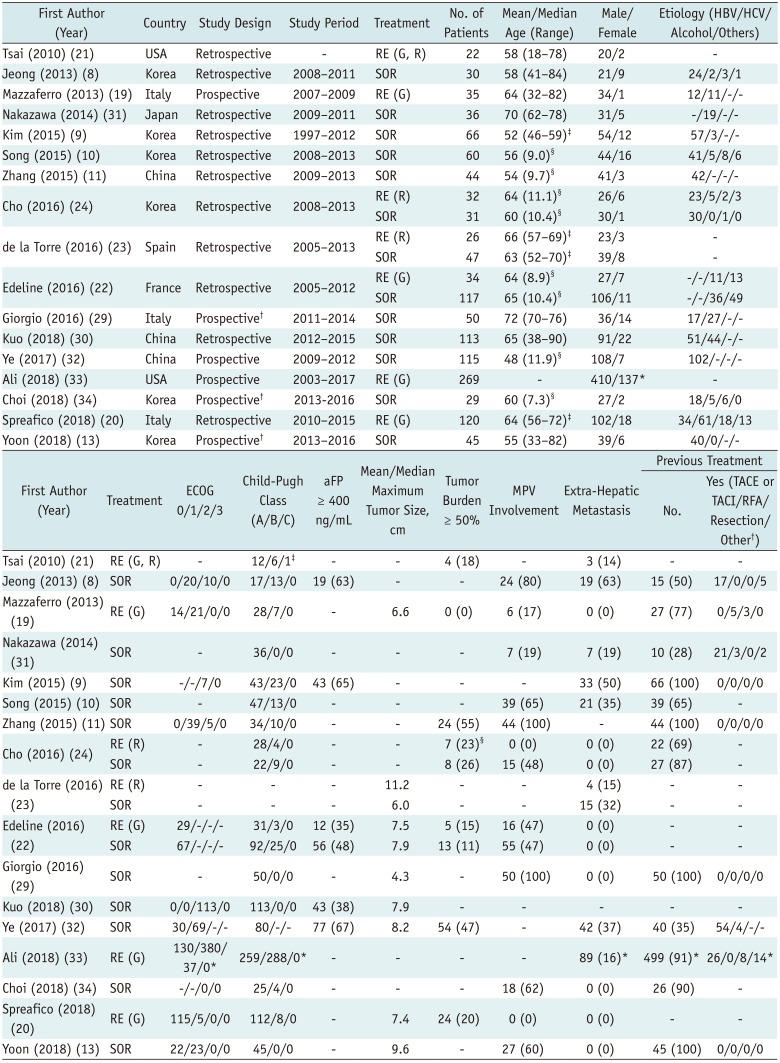
| First Author (Year) | Country | Study Design | Study Period | Treatment | No. of Patients | Mean/Median Age (Range) | Male/Female | Etiology (HBV/HCV/ Alcohol/Others) |
|---|---|---|---|---|---|---|---|---|
| Tsai (2010) (21) | USA | Retrospective | - | RE (G, R) | 22 | 58 (18–78) | 20/2 | - |
| Jeong (2013) (8) | Korea | Retrospective | 2008–2011 | SOR | 30 | 58 (41–84) | 21/9 | 24/2/3/1 |
| Mazzaferro (2013) (19) | Italy | Prospective | 2007–2009 | RE (G) | 35 | 64 (32–82) | 34/1 | 12/11/-/- |
| Nakazawa (2014) (31) | Japan | Retrospective | 2009–2011 | SOR | 36 | 70 (62–78) | 31/5 | -/19/-/- |
| Kim (2015) (9) | Korea | Retrospective | 1997–2012 | SOR | 66 | 52 (46–59)‡ | 54/12 | 57/3/-/- |
| Song (2015) (10) | Korea | Retrospective | 2008–2013 | SOR | 60 | 56 (9.0)§ | 44/16 | 41/5/8/6 |
| Zhang (2015) (11) | China | Retrospective | 2009–2013 | SOR | 44 | 54 (9.7)§ | 41/3 | 42/-/-/- |
| Cho (2016) (24) | Korea | Retrospective | 2008–2013 | RE (R) | 32 | 64 (11.1)§ | 26/6 | 23/5/2/3 |
| SOR | 31 | 60 (10.4)§ | 30/1 | 30/0/1/0 | ||||
| de la Torre (2016) (23) | Spain | Retrospective | 2005–2013 | RE (R) | 26 | 66 (57–69)‡ | 23/3 | - |
| SOR | 47 | 63 (52–70)‡ | 39/8 | - | ||||
| Edeline (2016) (22) | France | Retrospective | 2005–2012 | RE (G) | 34 | 64 (8.9)§ | 27/7 | -/-/11/13 |
| SOR | 117 | 65 (10.4)§ | 106/11 | -/-/36/49 | ||||
| Giorgio (2016) (29) | Italy | Prospective† | 2011–2014 | SOR | 50 | 72 (70–76) | 36/14 | 17/27/-/- |
| Kuo (2018) (30) | China | Retrospective | 2012–2015 | SOR | 113 | 65 (38–90) | 91/22 | 51/44/-/- |
| Ye (2017) (32) | China | Prospective | 2009–2012 | SOR | 115 | 48 (11.9)§ | 108/7 | 102/-/-/- |
| Ali (2018) (33) | USA | Prospective | 2003–2017 | RE (G) | 269 | - | 410/137* | - |
| Choi (2018) (34) | Korea | Prospective† | 2013-2016 | SOR | 29 | 60 (7.3)§ | 27/2 | 18/5/6/0 |
| Spreafico (2018) (20) | Italy | Retrospective | 2010–2015 | RE (G) | 120 | 64 (56–72)‡ | 102/18 | 34/61/18/13 |
| Yoon (2018) (13) | Korea | Prospective† | 2013–2016 | SOR | 45 | 55 (33–82) | 39/6 | 40/0/-/- |
| First Author (Year) | Treatment | ECOG 0/1/2/3 | Child-Pugh Class (A/B/C) | aFP ≥ 400 ng/mL | Mean/Median Maximum Tumor Size, cm | Tumor Burden ≥ 50% | MPV Involvement | Extra-Hepatic Metastasis | Previous Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Yes (TACE or TACI/RFA/Resection/Other†) | |||||||||
| Tsai (2010) (21) | RE (G, R) | - | 12/6/1‡ | - | - | 4 (18) | - | 3 (14) | - | - |
| Jeong (2013) (8) | SOR | 0/20/10/0 | 17/13/0 | 19 (63) | - | - | 24 (80) | 19 (63) | 15 (50) | 17/0/0/5 |
| Mazzaferro (2013) | RE (G) (19) | 14/21/0/0 | 28/7/0 | - | 6.6 | 0 (0) | 6 (17) | 0 (0) | 27 (77) | 0/5/3/0 |
| Nakazawa (2014) | SOR (31) | - | 36/0/0 | - | - | - | 7 (19) | 7 (19) | 10 (28) | 21/3/0/2 |
| Kim (2015) (9) | SOR | -/-/7/0 | 43/23/0 | 43 (65) | - | - | - | 33 (50) | 66 (100) | 0/0/0/0 |
| Song (2015) (10) | SOR | - | 47/13/0 | - | - | - | 39 (65) | 21 (35) | 39 (65) | - |
| Zhang (2015) (11) | SOR | 0/39/5/0 | 34/10/0 | - | - | 24 (55) | 44 (100) | - | 44 (100) | 0/0/0/0 |
| Cho (2016) (24) | RE (R) | - | 28/4/0 | - | - | 7 (23)§ | 0 (0) | 0 (0) | 22 (69) | - |
| SOR | - | 22/9/0 | - | - | 8 (26) | 15 (48) | 0 (0) | 27 (87) | - | |
| de la Torre (2016) (23) | RE (R) | - | - | - | 11.2 | - | - | 4 (15) | - | - |
| SOR | - | - | - | 6.0 | - | - | 15 (32) | - | - | |
| Edeline (2016) (22) | RE (G) | 29/-/-/- | 31/3/0 | 12 (35) | 7.5 | 5 (15) | 16 (47) | 0 (0) | - | - |
| SOR | 67/-/-/- | 92/25/0 | 56 (48) | 7.9 | 13 (11) | 55 (47) | 0 (0) | - | - | |
| Giorgio (2016) (29) | SOR | - | 50/0/0 | - | 4.3 | - | 50 (100) | 0 (0) | 50 (100) | 0/0/0/0 |
| Kuo (2018) (30) | SOR | 0/0/113/0 | 113/0/0 | 43 (38) | 7.9 | - | - | - | - | - |
| Ye (2017) (32) | SOR | 30/69/-/- | 80/-/- | 77 (67) | 8.2 | 54 (47) | - | 42 (37) | 40 (35) | 54/4/-/- |
| Ali (2018) (33) | RE (G) | 130/380/ | 259/288/0* | - | - | - | - | 89 (16)* | 499 (91)* | 26/0/8/14 |
| Choi (2018) (34) | SOR | -/-/0/0 | 25/4/0 | - | - | - | 18 (62) | 0 (0) | 26 (90) | - |
| Spreafico (2018) (20) | RE (G) | 115/5/0/0 | 112/8/0 | - | 7.4 | 24 (20) | 0 (0) | 0 (0) | - | - |
| Yoon (2018) (13) | SOR | 22/23/0/0 | 45/0/0 | - | 9.6 | - | 27 (60) | 0 (0) | 45 (100) | 0/0/0/0 |
*Data only available for entire study population only, †Randomized controlled trial, ‡Interquartile range, §Standard deviation. G = glass, HBV = hepatitis B virus, HCV = hepatitis C virus, R = resin, RCT= randomized controlled trial, RE = radioembolization, SOR = sorafenib
Data are shown as n (%). *Data available for entire study population only, †Including radiotherapy and liver transplantation, ‡Information was not available in three patients, §Information was not available in one patient. aFP = alpha-fetoprotein, ECOG = Eastern Cooperative Oncology Group, MPV = main portal vein, RFA = radiofrequency ablation, TACE = transcatheter arterial chemoembolization, TACI = transcatheter arterial chemotherapy infusion
Study Quality
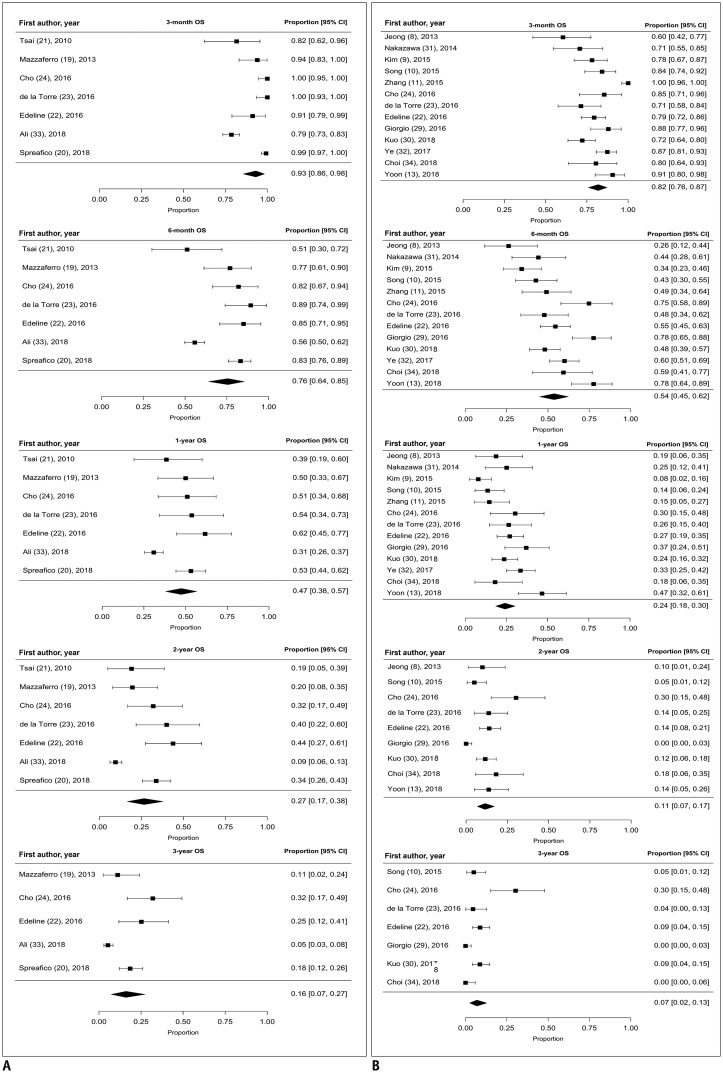
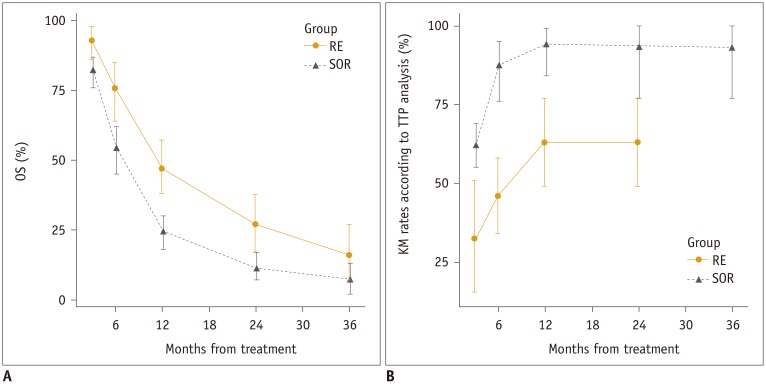
OS: Meta-Analysis
TTP: Meta-Analysis
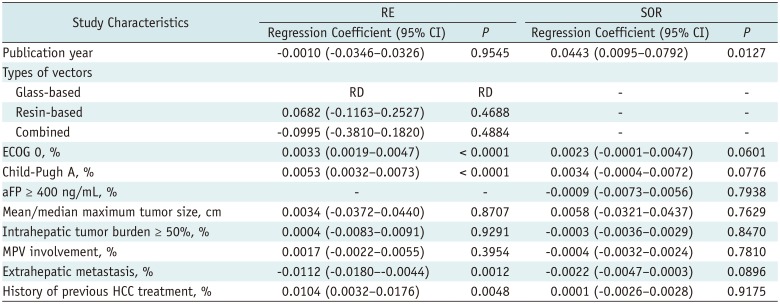
Meta-Regression Analysis for 1-Year OS
Table 2
Summary of Meta-Regression Analyses of 1-Year Overall Survival Data
AEs
Table 3
Summary of Adverse Events Following RE
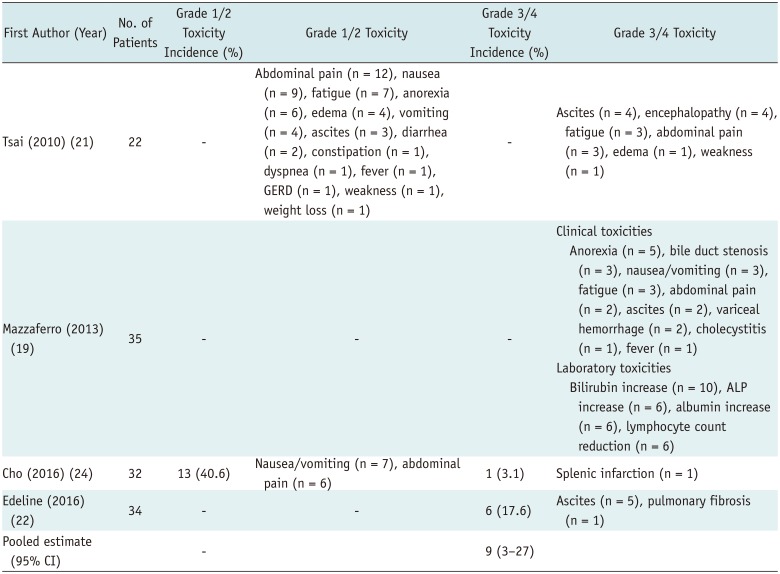
| First Author (Year) | No. of Patients | Grade 1/2 Toxicity Incidence (%) | Grade 1/2 Toxicity | Grade 3/4 Toxicity Incidence (%) | Grade 3/4 Toxicity |
|---|---|---|---|---|---|
| Tsai (2010) (21) | 22 | - | Abdominal pain (n = 12), nausea (n = 9), fatigue (n = 7), anorexia (n = 6), edema (n = 4), vomiting (n = 4), ascites (n = 3), diarrhea (n = 2), constipation (n = 1), dyspnea (n = 1), fever (n = 1), GERD (n = 1), weakness (n = 1), weight loss (n = 1) | - | Ascites (n = 4), encephalopathy (n = 4), fatigue (n = 3), abdominal pain (n = 3), edema (n = 1), weakness (n = 1) |
| Mazzaferro (2013) (19) | 35 | - | - | - | Clinical toxicities Anorexia (n = 5), bile duct stenosis (n = 3), nausea/vomiting (n = 3), fatigue (n = 3), abdominal pain (n = 2), ascites (n = 2), variceal hemorrhage (n = 2), cholecystitis (n = 1), fever (n = 1) Laboratory toxicities Bilirubin increase (n = 10), ALP increase (n = 6), albumin increase (n = 6), lymphocyte count reduction (n = 6) |
| Cho (2016) (24) | 32 | 13 (40.6) | Nausea/vomiting (n = 7), abdominal pain (n = 6) | 1 (3.1) | Splenic infarction (n = 1) |
| Edeline (2016) (22) | 34 | - | - | 6 (17.6) | Ascites (n = 5), pulmonary fibrosis (n = 1) |
| Pooled estimate (95% CI) | - | 9 (3–27) |
Adverse events were defined and categorized in accordance with NCI-CTCAE version 5.0; meta-analytic pooled estimates were based on inverse variance method for calculating weights with random-effects model. ALP = alkaline phosphatase, GERD = gastroesophageal reflux disease, NCI-CTCAE = National Cancer Institute-Common Terminology Criteria for Adverse Events
Table 4
Summary of Adverse Events Associated with SOR Treatment
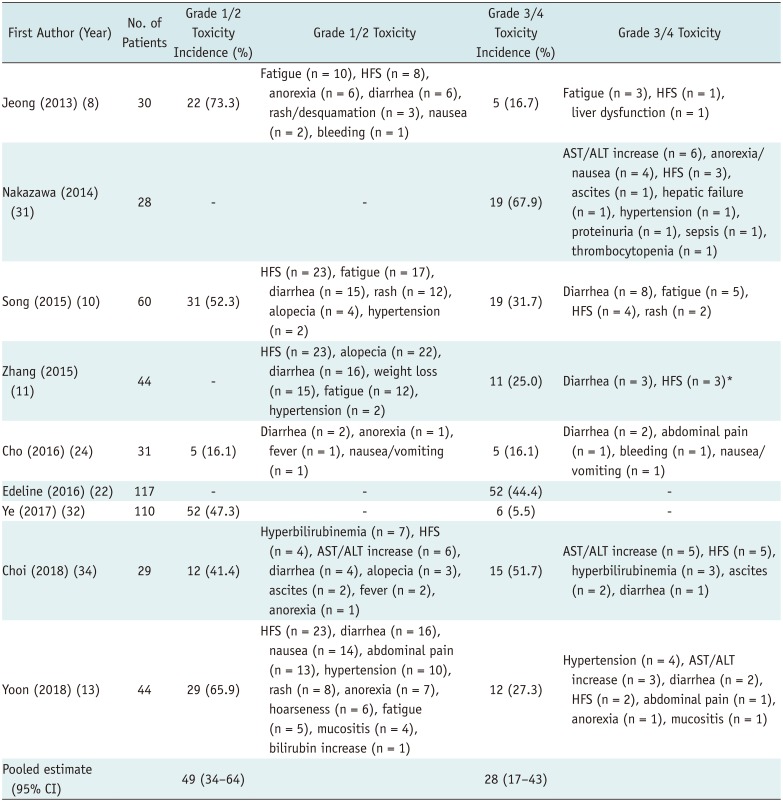
| First Author (Year) | No. of Patients | Grade 1/2 Toxicity Incidence (%) | Grade 1/2 Toxicity | Grade 3/4 Toxicity Incidence (%) | Grade 3/4 Toxicity |
|---|---|---|---|---|---|
| Jeong (2013) (8) | 30 | 22 (73.3) | Fatigue (n = 10), HFS (n = 8), anorexia (n = 6), diarrhea (n = 6), rash/desquamation (n = 3), nausea (n = 2), bleeding (n = 1) | 5 (16.7) | Fatigue (n = 3), HFS (n = 1), liver dysfunction (n = 1) |
| Nakazawa (2014) (31) | 28 | - | - | 19 (67.9) | AST/ALT increase (n = 6), anorexia/nausea (n = 4), HFS (n = 3), ascites (n = 1), hepatic failure (n = 1), hypertension (n = 1), proteinuria (n = 1), sepsis (n = 1), thrombocytopenia (n = 1) |
| Song (2015) (10) | 60 | 31 (52.3) | HFS (n = 23), fatigue (n = 17), diarrhea (n = 15), rash (n = 12), alopecia (n = 4), hypertension (n = 2) | 19 (31.7) | Diarrhea (n = 8), fatigue (n = 5), HFS (n = 4), rash (n = 2) |
| Zhang (2015) (11) | 44 | - | HFS (n = 23), alopecia (n = 22), diarrhea (n = 16), weight loss (n = 15), fatigue (n = 12), hypertension (n = 2) | 11 (25.0) | Diarrhea (n = 3), HFS (n = 3)* |
| Cho (2016) (24) | 31 | 5 (16.1) | Diarrhea (n = 2), anorexia (n = 1), fever (n = 1), nausea/vomiting (n = 1) | 5 (16.1) | Diarrhea (n = 2), abdominal pain (n = 1), bleeding (n = 1), nausea/vomiting (n = 1) |
| Edeline (2016) (22) | 117 | - | - | 52 (44.4) | - |
| Ye (2017) (32) | 110 | 52 (47.3) | - | 6 (5.5) | - |
| Choi (2018) (34) | 29 | 12 (41.4) | Hyperbilirubinemia (n = 7), HFS (n = 4), AST/ALT increase (n = 6), diarrhea (n = 4), alopecia (n = 3), ascites (n = 2), fever (n = 2), anorexia (n = 1) | 15 (51.7) | AST/ALT increase (n = 5), HFS (n = 5), hyperbilirubinemia (n = 3), ascites (n = 2), diarrhea (n = 1) |
| Yoon (2018) (13) | 44 | 29 (65.9) | HFS (n = 23), diarrhea (n = 16), nausea (n = 14), abdominal pain (n = 13), hypertension (n = 10), rash (n = 8), anorexia (n = 7), hoarseness (n = 6), fatigue (n = 5), mucositis (n = 4), bilirubin increase (n = 1) | 12 (27.3) | Hypertension (n = 4), AST/ALT increase (n = 3), diarrhea (n = 2), HFS (n = 2), abdominal pain (n = 1), anorexia (n = 1), mucositis (n = 1) |
| Pooled estimate (95% CI) | 49 (34–64) | 28 (17–43) |
*Details of adverse events were incomplete in study. Adverse events were defined and categorized in accordance with NCI-CTCAE version 5.0; meta-analytic pooled estimates were based on inverse variance method for calculating weights with random-effects model. ALT = alanine aminotransferase, AST = aspartate aminotransferase, HFS = hand-foot syndrome




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download