1. McNeely CA, Vassileva CM. Long-term outcomes of mitral valve repair versus replacement for degenerative disease: a systematic review. Curr Cardiol Rev. 2015; 11:157–162. PMID:
25158683.

2. Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007; 31:267–275. PMID:
17175161.

3. Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017; 135:410–422. PMID:
27899396.
4. Gammie JS, Sheng S, Griffith BP, Peterson ED, Rankin JS, O'Brien SM, et al. Trends in mitral valve surgery in the United States: results from the society of thoracic surgeons adult cardiac surgery database. Ann Thorac Surg. 2009; 87:1431–1437. discussion 1437-1439. PMID:
19379881.
5. Bolling SF, Li S, O'Brien SM, Brennan JM, Prager RL, Gammie JS. Predictors of mitral valve repair: clinical and surgeon factors. Ann Thorac Surg. 2010; 90:1904–1911. discussion 1912. PMID:
21095334.

6. Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012; 144:308–312. PMID:
22698565.

7. David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg. 2005; 130:1242–1249. PMID:
16256774.

8. Mihaljevic T, Jarrett CM, Gillinov AM, Williams SJ, DeVilliers PA, Stewart WJ, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg. 2011; 141:72–80. e1–e4. PMID:
21093881.

9. David TE. Durability of mitral valve repair for mitral regurgitation due to degenerative mitral valve disease. Ann Cardiothorac Surg. 2015; 4:417–421. PMID:
26539345.
10. Gillinov AM, Cosgrove DM, Blackstone EH, Diaz R, Arnold JH, Lytle BW, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998; 116:734–743. PMID:
9806380.

11. David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013; 127:1485–1492. PMID:
23459614.

12. Oliveira JM, Antunes MJ. Mitral valve repair: better than replacement. Heart. 2006; 92:275–281. PMID:
16415204.

13. Fusini L, Ghulam Ali S, Tamborini G, Muratori M, Gripari P, Maffessanti F, et al. Prevalence of calcification of the mitral valve annulus in patients undergoing surgical repair of mitral valve prolapse. Am J Cardiol. 2014; 113:1867–1873. PMID:
24837266.

14. Okada Y. Surgical management of mitral annular calcification. Gen Thorac Cardiovasc Surg. 2013; 61:619–625. PMID:
23404309.

15. Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996; 111:718–729. discussion 729-730. PMID:
8614132.

16. Uchimuro T, Fukui T, Shimizu A, Takanashi S. Mitral valve surgery in patients with severe mitral annular calcification. Ann Thorac Surg. 2016; 101:889–895. PMID:
26545623.
17. Gillinov AM, Cosgrove DM, Lytle BW, Taylor PC, Stewart RW, McCarthy PM, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg. 1997; 113:467–473. discussion 473-475. PMID:
9081091.

18. DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg. 2010; 139:76–83. discussion 83-84. PMID:
19931098.

19. Suri RM, Clavel MA, Schaff HV, Michelena HI, Huebner M, Nishimura RA, et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: long-term analysis of competing outcomes. J Am Coll Cardiol. 2016; 67:488–498. PMID:
26846946.
20. Northrup WF 3rd, DuBois KA, Kshettry VR. Morbidity and mortality of a failed attempt at mitral valve repair converted to replacement at the same operation. J Heart Valve Dis. 2003; 12:700–706. PMID:
14658808.
21. Ben Zekry S, Nagueh SF, Little SH, Quinones MA, McCulloch ML, Karanbir S, et al. Comparative accuracy of two- and three-dimensional transthoracic and transesophageal echocardiography in identifying mitral valve pathology in patients undergoing mitral valve repair: initial observations. J Am Soc Echocardiogr. 2011; 24:1079–1085. PMID:
21803543.

22. Kim JH, Kim EY, Jin GY, Choi JB. A review of the use of cardiac computed tomography for evaluating the mitral valve before and after mitral valve repair. Korean J Radiol. 2017; 18:773–785. PMID:
28860895.

23. Ghosh N, Al-Shehri H, Chan K, Mesana T, Chan V, Chen L, et al. Characterization of mitral valve prolapse with cardiac computed tomography: comparison to echocardiographic and intraoperative findings. Int J Cardiovasc Imaging. 2012; 28:855–863. PMID:
21604082.

24. Feuchtner GM, Alkadhi H, Karlo C, Sarwar A, Meier A, Dichtl W, et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography1. Radiology. 2010; 254:374–383. PMID:
20093510.
25. Smith T, Gurudevan S, Cheng V, Trento A, Derobertis M, Thomson L, et al. Assessment of the morphological features of degenerative mitral valve disease using 64-slice multi detector computed tomography. J Cardiovasc Comput Tomogr. 2012; 6:415–421. PMID:
23146347.

26. Shah RG, Novaro GM, Blandon RJ, Wilkinson L, Asher CR, Kirsch J. Mitral valve prolapse: evaluation with ECG-gated cardiac CT angiography. AJR Am J Roentgenol. 2010; 194:579–584. PMID:
20173131.

27. Delgado V, Tops LF, Schuijf JD, de Roos A, Brugada J, Schalij MJ, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging. 2009; 2:556–565. PMID:
19442940.

28. Ng C, Nesser J, Punzengruber J, Pandian NG, Khandheria B, Hartl P, et al. Modern mitral valve repair: echocardiographic interpretations and surgical strategies. New York, NY: Springer Verlag;2003. p. 29–87.
29. Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg. 1983; 86:323–337. PMID:
6887954.

30. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832. PMID:
2407762.

31. Silaschi M, Chaubey S, Aldalati O, Khan H, Uzzaman MM, Singh M, et al. Is mitral valve repair superior to mitral valve replacement in elderly patients? Comparison of short- and long-term outcomes in a propensity-matched cohort. J Am Heart Assoc. 2016; 5:pii: e003605.

32. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270. PMID:
25712077.

33. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981; 64:744–753. PMID:
7273375.

34. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. J Am Soc Echocardiogr. 2003; 16:777–802. PMID:
12835667.

35. Kim JH, Lee SH, Joo HC, Youn YN, Yoo KJ, Chang BC, et al. Effect of recurrent mitral regurgitation after mitral valve repair in patients with degenerative mitral regurgitation. Circ J. 2017; 82:93–101. PMID:
28724839.

36. Labovitz AJ, Nelson JG, Windhorst DM, Kennedy HL, Williams GA. Frequency of mitral valve dysfunction from mitral anular calcium as detected by doppler echocardiography. Am J Cardiol. 1985; 55:133–137. PMID:
3966372.

37. Movahed MR, Saito Y, Ahmadi-Kashani M, Ebrahimi R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound. 2007; 5:14. PMID:
17359540.

38. Muddassir SM, Pressman GS. Mitral annular calcification as a cause of mitral valve gradients. Int J Cardiol. 2007; 123:58–62. PMID:
17320214.

39. Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J. 1984; 107:989–996. PMID:
6372421.

40. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ, et al. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285. PMID:
25741189.

41. Pontone G, Andreini D, Bertella E, Cortinovis S, Mushtaq S, Foti C, et al. Pre-operative CT coronary angiography in patients with mitral valve prolapse referred for surgical repair: comparison of accuracy, radiation dose and cost versus invasive coronary angiography. Int J Cardiol. 2013; 167:2889–2894. PMID:
22959395.

42. Morris MF, Suri RM, Akhtar NJ, Young PM, Gruden JF, Burkhart HM, et al. Computed tomography as an alternative to catheter angiography prior to robotic mitral valve repair. Ann Thorac Surg. 2013; 95:1354–1359. PMID:
23394969.

43. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014; 129:e521–e643. PMID:
24589853.

44. Higgins J, Mayo J, Skarsgard P. Cardiac computed tomography facilitates operative planning in patients with mitral calcification. Ann Thorac Surg. 2013; 95:e9–e11. PMID:
23272892.

45. Hamirani YS, Nasir K, Blumenthal RS, Takasu J, Shavelle D, Kronmal R, et al. Relation of mitral annular calcium and coronary calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2011; 107:1291–1294. PMID:
21349485.

46. Killeen RP, Arnous S, Martos R, Abbara S, Quinn M, Dodd JD. Chronic mitral regurgitation detected on cardiac MDCT: differentiation between functional and valvular aetiologies. Eur Radiol. 2010; 20:1886–1895. PMID:
20309557.

47. Moodley S, Schoenhagen P, Gillinov AM, Mihaljevic T, Flamm SD, Griffin BP, et al. Preoperative multidetector computed tomograpy angiography for planning of minimally invasive robotic mitral valve surgery: impact on decision making. J Thorac Cardiovasc Surg. 2013; 146:262–268.e1. PMID:
22841167.

48. Blanke P, Dvir D, Cheung A, Ye J, Levine RA, Precious B, et al. A simplified D-shaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J Cardiovasc Comput Tomogr. 2014; 8:459–467. PMID:
25467833.

49. Blanke P, Naoum C, Webb J, Dvir D, Hahn RT, Grayburn P, et al. Multimodality imaging in the context of transcatheter mitral valve replacement: establishing consensus among modalities and disciplines. JACC Cardiovasc Imaging. 2015; 8:1191–1208. PMID:
26481845.
50. Mak GJ, Blanke P, Ong K, Naoum C, Thompson CR, Webb JG, et al. Three-dimensional echocardiography compared with computed tomography to determine mitral annulus size before transcatheter mitral valve implantation. Circ Cardiovasc Imaging. 2016; 9:pii: e004176.

51. Naoum C, Leipsic J, Cheung A, Ye J, Bilbey N, Mak G, et al. Mitral annular dimensions and geometry in patients with functional mitral regurgitation and mitral valve prolapse: implications for transcatheter mitral valve implantation. JACC Cardiovasc Imaging. 2016; 9:269–280. PMID:
26897676.
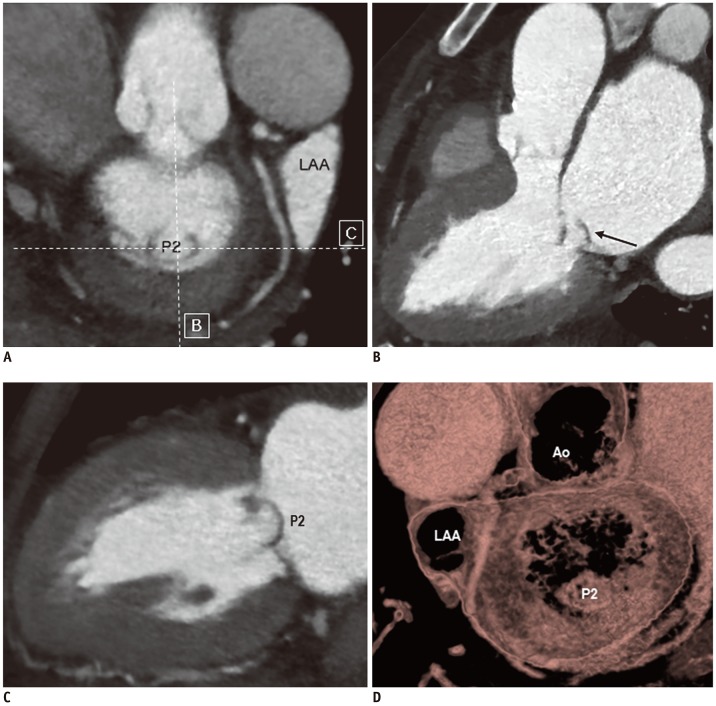


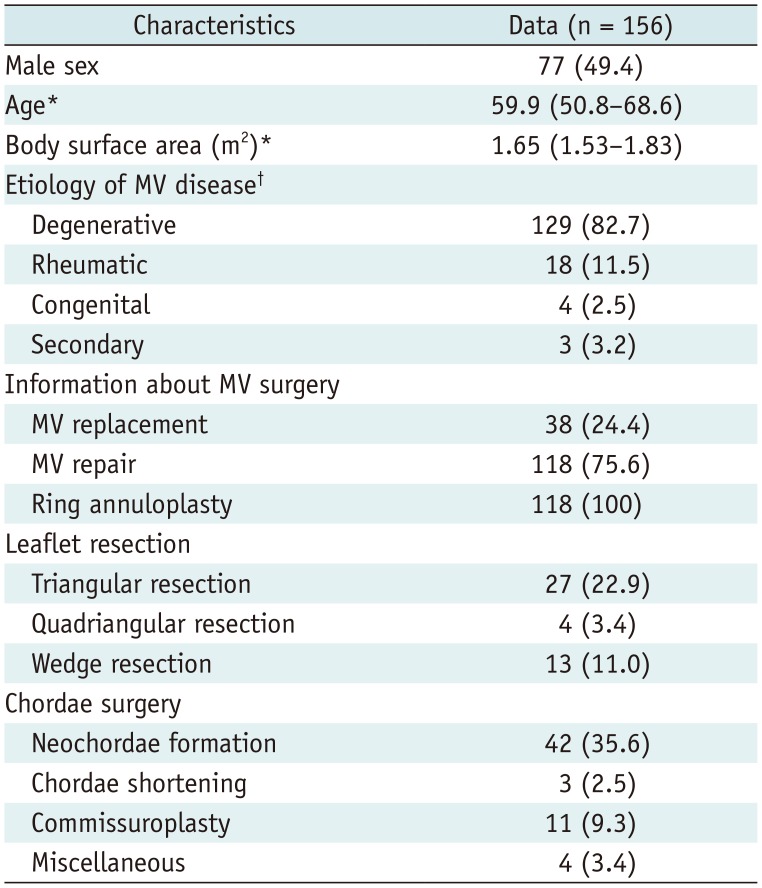
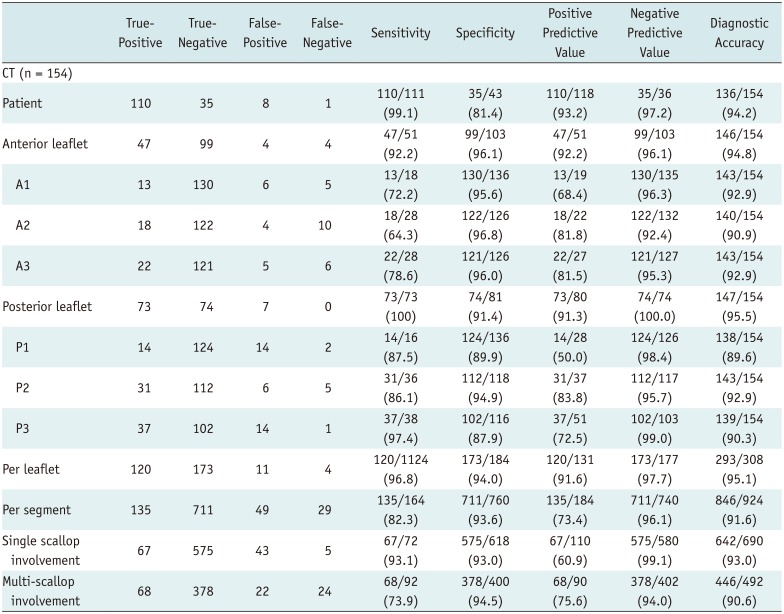
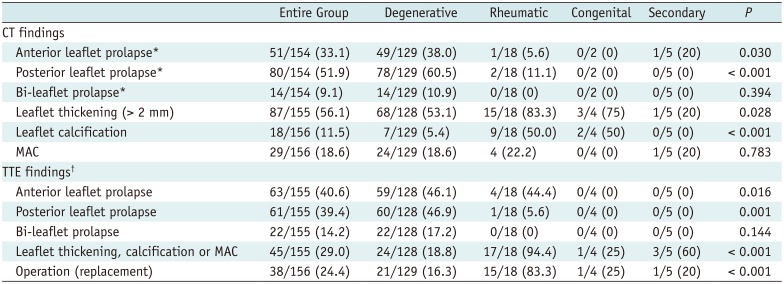




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download