INTRODUCTION
Ovarian sex cord-stromal tumors (SCSTs) is a heterogenous group of tumors that arise from primitive sex cord or stromal cells. They account for approximately 7% of all ovarian tumors [
1]. Patients usually present with stage I disease and have an excellent long-term prognosis [
12]. The most common histologic subtype is granulosa cell (GC) tumor while non-GC tumors such as Sertoli-Leydig cell, steroid cell tumors, gynandroblastoma and SCST with annular tubules are exceedingly rare [
2]. Based on existing data Sertoli-Leydig cell tumors (SLCTs) comprise less than 0.5% of all ovarian tumors, while steroid cell tumors account for less than 0.1% of all ovarian tumors [
34]. Given the rarity of non-GC SCSTs, current evidence derives from small single institutional studies, spanning over multiple years [
3456789101112]. Inevitably, the management of patients with non-GC SCSTs is extrapolated from those with GC or germ-cell tumors. While patients with advanced stage disease routinely receive chemotherapy (CT) following debulking surgery, to date a benefit of adjuvant treatment for those with early stage SCSTs has yet to be demonstrated. Currently, the National Comprehensive Cancer Network (NCCN) guidelines suggest that adjuvant CT can be considered for patients with stage IC disease, poorly differentiated tumors or in the presence of heterologous elements [
13]. Similarly, the recently updated European Society for Medical Oncology (ESMO) guidelines on the management of non-epithelial ovarian tumors recommend the administration of adjuvant CT for patients with SLCTs in the presence of heterologous elements, poor tumor differentiation or stage IB disease and above [
14]. The aim of the present study was to evaluate the role of adjuvant CT in the management of patients with non-GC SCSTs, using a large multi-institutional, hospital-based database.
MATERIALS AND METHODS
The U.S National Cancer Data Base (NCDB) was accessed and patients diagnosed between 2004–2013 with a pathologically confirmed non-GC ovarian SCST were identified. Patients who did not undergo surgical treatment, those without information on the administration of CT or with less than one month of follow-up were excluded. The NCDB, has been established jointly by the American Cancer Society and Commission on Cancer of the American College of Surgeons, as a hospital-based database capturing approximately 70% of all newly diagnosed malignancies in the United States [
15]. Patient data are prospectively collected from participating commission-accredited cancer programs and are frequently audited to ensure their robustness. All data are de-identified and available for research purposes. Based on the NCDB data use agreement rules in an effort to maintain the anonymity of patients, cells with number <10 are suppressed. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytical or statistical methodology employed, or the conclusions drawn from these data.
Demographic and clinical information were extracted from the de-identified NCDB dataset. For analyses purposes patient race was recoded as White and non-White, age was dichotomized into <50 and ≥50 years and tumor size (when available) was categorized into <10 cm and ≥10 cm. Tumor grade (when available) was recoded into low-grade that included well (grade 1) and moderately (grade 2) differentiated tumors and high-grade than included poorly differentiated (grade 3) and undifferentiated (grade 4) tumors. Year of diagnosis was categorized into 2004–2006, 2007–2009, and 2010–2013. As in previous NCDB analyses, staging information was based on the pathological stage but if unknown the clinical stage was used [
16]. Demographic and clinico-pathological characteristics were compared with the χ
2 and Mann-Whitney U tests. Binary logistic regression was also performed to identify factors independently associated with the administration of adjuvant CT.
In the NCDB overall survival (OS) is defined as the number of months elapsed from tumor diagnosis to the date of death or last-follow-up. OS was evaluated following generation of Kaplan-Meier curves and compared with the log-rank test. Analysis was stratified by early and advanced stage disease. A Cox multivariate model was constructed to evaluate survival after controlling for tumor histology. All statistical analysis was performed with the SPSS v.24 statistical package (IBM Corp., Armonk, NY, USA), and the alpha level of statistical significance was set at 0.05.
RESULTS
A total of 391 patients met the inclusion criteria. According to the reverse Kaplan-Meier method, median follow-up of patients was 55.2 months. Median patient age was 39 years (interquartile range [IQR]=35) while the majority were of White race (71.3%) and presence of co-morbidities (Charlson/Deyo co-morbidity score ≥1) was infrequent (18.4%). The most common histologic subtype was SLCT (n=286, 73.1%,) followed by steroid cell carcinoma (n=47, 12%), Sertoli cell carcinoma (n=24, 6.1%), malignant thecoma (n=13, 3.3%), Leydig cell carcinoma (n=11, 2.8%), and other (n=10, 2.5%,)
Table 1 summarizes the histologic subtypes included in the study. Tumor grade was available for 304 patients, and 80.9% had high-grade tumors. Staging information was available for 343 patients, and the majority (n=291, 84.8%) had early stage disease (stage I–II).
Table 1
Histologic subtypes of patients included in the present study

|
Histotype |
ICD-O-3 code |
No. of cases |
|
Sertoli-Leydig cell carcinoma |
8631, 8633, 8634 |
286 (73.1) |
|
Sertoli cell carcinoma |
8640 |
24 (6.1) |
|
Steroid cell carcinoma |
8670 |
47 (12) |
|
Thecoma, malignant |
8600 |
13 (3.3) |
|
Leydig cell carcinoma |
8650 |
11 (2.8) |
|
Other |
8591, 8592, 8593, 8623, 8632 |
10 (2.5) |

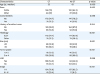
Rate of adjuvant CT administration was 51.9% (203/391) while median interval between surgery and CT administration was 39 days (n=195, IQR=28 days). Based on available information, the majority (96.3%) received a multiagent regimen. By univariate analysis patients who received CT were younger (median age 35 vs. 43 years, p=0.009), more likely to be of White race (78% vs. 64.2%, p=0.003), present with advanced stage disease (23.4% vs. 6.5%, p<0.001), have bilateral tumors (5.1% vs. 1.1%, p=0.037), larger than 10 cm in size (66.1% vs. 48.1%, p=0.001) of Sertoli-Leydig cell histology (84.7% vs. 60.6%, p<0.001) and higher tumor grade (64.2% vs. 31%, p<0.001). No difference in CT rates was noted based on year of diagnosis (p=0.98), patient median income (p=0.52), insurance type (p=0.23), type of reporting facility (p=0.38) and presence of medical co-morbidities (p=0.096) or history of another tumor (p=0.55) (
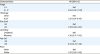
Table 2). By multivariate analysis advanced stage (odds ratio [OR]=4.65; p=0.001), SLCT histology (OR=3.51; p<0.001), White race (OR=1.87; p=0.035) and tumor size >10 cm (OR=1.83; p=0.025) were independently associated with the administration of CT (
Table 3).
Table 2
Clinico-pathological characteristics of patients with non-granulosa SCST stratified by administration of CT
|
Characteristics |
CT |
No CT |
p-value |
|
Age (yr, median) |
35 |
43 |
0.009 |
|
Race |
|
|
0.003 |
|
White |
156 (78) |
120 (64.2) |
|
Other |
44 (22) |
67 (35.8) |
|
Comorbidities |
|
|
0.096 |
|
No |
172 (84.7) |
147 (78.2) |
|
Yes |
31 (15.3) |
41 (21.8) |
|
History of another tumor |
|
|
0.554 |
|
No |
181 (89.2) |
164 (87.2) |
|
Yes |
22 (10.8) |
24 (12.8) |
|
Histology |
|
|
<0.001 |
|
SLCT |
172 (84.7) |
114 (60.6) |
|
Other |
31 (15.3) |
74 (39.4) |
|
Size (cm) |
|
|
0.001 |
|
≤10 |
59 (33.9) |
82 (51.9) |
|
>10 |
115 (66.1) |
76 (48.1) |
|
Tumor grade |
|
|
<0.001 |
|
1 or 2 |
18 (10.2) |
40 (31.3) |
|
3 or 4 |
158 (89.8) |
88 (68.8) |
|
LND |
|
|
0.066 |
|
Yes |
108 (54.3) |
84 (44.9) |
|
No |
91 (45.7) |
103 (55.1) |
|
Stage |
|
|
<0.001 |
|
I–II |
134 (76.6) |
157 (93.5) |
|
III–IV |
41 (23.4) |
11 (6.5) |

Table 3
Predictors of CT use in patients with non-granulosa SCSTs
|
Characteristics |
HR (95% CI) |
|
Stage |
|
|
I–II |
Ref. |
|
III–IV |
4.65 (1.92–11.23) |
|
Histology |
|
|
Other |
Ref. |
|
SLCT |
3.51 (1.91–6.45) |
|
Size (cm) |
|
|
<10 |
Ref. |
|
≥10 |
1.83 (1.08–3.10) |
|
Laterality |
|
|
Unilateral |
Ref. |
|
Bilateral |
1.36 (0.17–11.10) |
|
Age (yr) |
|
|
<50 |
Ref. |
|
≥50 |
0.63 (0.37–1.06) |
|
Race |
|
|
Other |
Ref. |
|
White |
1.87 (1.05–3.35) |

Regarding the specific details of the surgical procedures performed, lymph node sampling/dissection was performed in half of patients (49.7%) while based on the pathology report, rate of lymph node metastasis was 3.7% (7/188). Rate of CT administration was higher among patients who had lymph node dissection (LND; 56.3% vs. 46.9%, p=0.07). The uterus was preserved in approximately half of patients (53.5%, 169/316), while rates of CT administration did not differ between patients who did (44.9%) and did not (48.5%) undergo hysterectomy, p=0.52. Information on residual disease status was available for 141 cases, 96.5% did not have any macroscopic residual tumor following surgery; all patients with macroscopic residual disease received CT.
For patients with early stage (I–II) disease, there was no difference in OS between those who received CT (n=134) and those who did not (n=157), p=0.40; 5-year OS rates were 81.7% and 84.6%, respectively (
Fig. 1). After controlling for histology (SLCT vs. non-SLCT), administration of CT was not associated with better mortality (hazard ratio [HR]=0.73; 95% confidence interval [CI]=0.38–1.40) for patients with early stage disease. When evaluated separetely, administration of CT (n=118) was not associated with better OS compared to observation (n=150) for patients with stage I disease (p=0.83; 5-year OS rates 85.1% and 85.7%, respectively). Similarly, administration of CT was not associated with a survival benefit for patients with stage II disease (n=23, p=0.25; 5-year OS rates were 75% and 54.5% in the observation and CT groups, respectively).
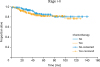 | Fig. 1
OS of patients with early stage (I–II) ovarian non-granulosa SCSTs who did (n=134) and did not (n=157) receive adjuvant chemotherapy, p=0.40 from log-rank test; 5-year OS rates 81.7% and 84.6%, respectively.
OS, overall survival; SCST, sex cord-stromal tumor.

|
Patients with advanced stage (III–IV) disease who received CT (n=41) had better OS compared to those who did not (n=11), p=0.013; median OS was 34.96 months (95% CI=10.64–59.28) and 15.51 months (95% CI=5.37–25.62), respectively (
Fig. 2). After controlling for histology (SLCT vs. non-SLCT), administration of CT was associated with better mortality (HR=0.40; 95% CI=0.19–0.85). Among the 11 patients with advanced stage disease who did not receive CT, the majority (90%) were not offered CT by the treating physician.
 | Fig. 2
OS of patients with advanced stage (III–IV) ovarian non-granulosa SCSTs who did (n=41) and did not (n=11) receive adjuvant chemotherapy, p=0.013 from log-rank test; median OS 34.96 and 15.51 months, respectively.
OS, overall survival; SCST, sex cord-stromal tumor.

|
For patients with low-grade tumors there was no difference in OS between those who received CT (n=18) and those who did not (n=40), p=0.33; 5-year OS rates were 75.1% and 79.6%, respectively. In addition, there was no OS difference between the observation (n=88) and CT (n=158) groups for patients with high-grade tumors, p=0.39; 5-year OS rates were 80.5% and 73%, respectively. When limiting the analysis to patients with early stage disease and high-grade tumors, there was no difference in OS between those who received CT (n=108) and those who did not (n=71), p=0.50; 5-year OS rates were 82.9% and 81.4%, respectively.
When evaluating patients with SLCTs with known tumor grade, those with low-grade tumors who received CT (n=11) did not have a statistically better survival than those who did not (n=11), p=0.28; 5-year OS were 68.6% and 87.5%, respectively. For patients with high-grade SLCTs there was no statistically significant difference in OS between the CT (n=149) and observation (n=87) groups, p=0.37; 5-year OS were 74.2% and 81.8%, respectively.
In the present cohort of patients with non-GC SCSTs, tumor size >10 cm (p=0.20), grade 3/4 (p=0.53), presence of medical co-morbidities (p=0.21), non-White race (p=0.81), age >50 years (p=0.24), and omission of LND (p=0.31) were not associated with a statistically worse OS by univariate analysis.
DISCUSSION
This is one of the largest cohort of patients diagnosed with malignant ovarian non-GC SCSTs presented in literature. Administration of adjuvant CT was not associated with a survival benefit for patients with early stage disease even for those with high-grade tumors indicating that surgical treatment alone may be sufficient. On the other hand, patients with advanced stage disease who received CT had better OS.
In our study, 84.8% of patients were diagnosed with early stage disease, consistent with existing literature. Approximately 70% of all SCSTs, including GC type, are diagnosed at stage I [
1]. In a retrospective study of 16 patients with Sertoli-Leydig tumors, 88% had stage IA disease [
5]. In another cohort 63 patients with steroid cell tumors, 87% were diagnosed with stage I or II disease [
4]. The role of LND in the staging of SCSTs is not well established. In our study, only 49.7% of patients underwent lymph node sampling/dissection. Rate of lymph node metastasis was low (3.8%), comparable to a previous population-based study (3.3%), while performance of LND was not associated with a survival benefit [
17]. Based on current evidence LND could be omitted in the absence of grossly abnormal lymph nodes.
Compared to GC tumors that are characterized by late recurrences even following 10 years from initial diagnosis, the majority of relapses for SLCTs occur within the first 5 years, especially in the presence of unfavorable tumor characteristics. In a large cohort of patients with SLCTs, disease free survival for patients with poorly differentiated tumors (n=22) was 41% while for those with poorly differentiated tumors with heterologous elements only 12.5% (n=8) [
12]. As such the NCCN and ESMO guidelines recommend the administration of CT for patients with poorly differentiated tumors, those who contain heterologous elements or for patients with advanced stage disease [
1314]. For patients with steroid tumors, the Gynecologic Inter Group recommend the administration of adjuvant CT for pleomorphic tumors, increased mitotic count, large, or advanced stage [
1]. Nevertheless, the efficacy of adjuvant CT for non-GC tumors is based on small retrospective studies. Most of them do not include a comparison to an observation arm or lack appropriate statistical power. In a retrospective cohort of 34 patients with intermediate or poorly differentiated SLCTs, 23 patients received adjuvant CT and none (0%) relapsed, compared to 2 (18.2%) patients in the observation group (n=11), p>0.05 [
9]. However, both recurrences were successfully salvaged after 4 cycles of bleomycin-etoposide-cisplatin (BEP). Interestingly, tumor relapse can occur even following the administration of adjuvant CT. In a Taiwanese Gynecologic Oncology Group study, a patient diagnosed with a poorly differentiated stage IA SLCT who underwent unilateral oophorectomy and received 4 cycles of BEP experienced a relapse 2 years after primary treatment. Secondary cytoreductive surgery followed by salvage CT were not successful and patient deceased [
7]. Bhat et al. [
8] also described a recurrence in a patient diagnosed a poorly differentiated stage IC SLCT who received BEP adjuvant CT. In our study we failed to demonstrate a survival benefit of adjuvant CT for patients diagnosed with early stage disease, even for those with grade 3 tumors. Adjuvant CT can be associated with significant morbidity and have detrimental effect on the quality of life of cancer survivors. Given the lack of a clear evidence on its benefit, the decision to administer CT should be individualized and made following extensive counseling also taking into consideration that tumor relapses for non-granulosa SCSTs are generally associated with poor outcomes [
318].
However, our findings suggest a possible clinical benefit of adjuvant CT for patients with advanced stage disease (median OS: 34.96 vs. 15.51 months, p=0.013). While major guidelines recommend the administration of CT in this patient group, evidence remains sparse regarding its clinical benefit. SCST tumors frequently exhibit resistance to CT and have high rates of recurrence [
19]. Rates of adjuvant CT response rate range from 63%–80% [
120]. Interestingly, in a previous analysis of the NCDB, no benefit of adjuvant CT was demonstrated for patients with advanced stage GC tumors (HR=0.80; 95% CI=0.52–1.23) [
21]. In our cohort survival for patients with advanced stage non-GC tumors was 24.9 months. Given the observed poor outcomes even with the use of CT the development of novel treatment options is greatly warranted.
Several limitations of the present study should be noted. Firstly, due to the lack of central pathology review tumor misclassifications cannot be excluded. The NCDB does not collect information on tumor recurrence or cause of death precluding us from analyzing differences in relapse rates, progression-free and cancer-specific survival. While this is the largest cohort of patients with non-GC ovarian tumors our study may have not been adequately powered to detect a statistically significant difference in OS in specific subgroup analyses. Also, even though almost all patients received a multi-agent regimen information on the exact composition and dosage was not available. Lastly, information on residual disease following debulking surgery an important prognostic factor, especially for patients with advanced stage disease, was available only for 141 patients.
International collaborations can further elucidate the optimal management of patients diagnosed with these rare tumors. Certain patients with early stage disease could potentially be spared from the administration of adjuvant CT.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download