Abstract
Objectives
Materials and Methods
References
Figure 1.
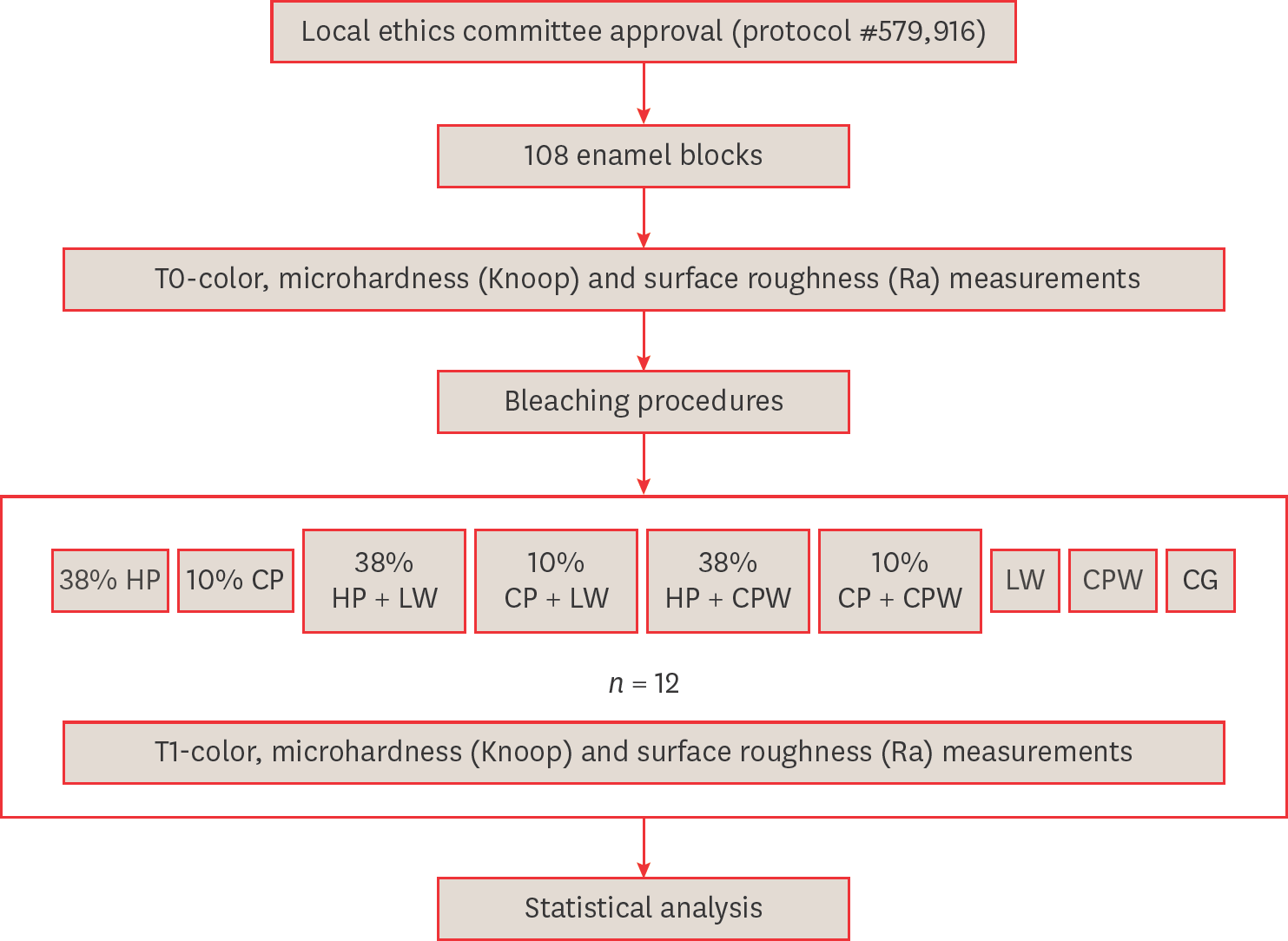
Table 1.
Table 2.
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
Table 3.
Data are presented as means ± standard deviations. Values with different superscript letters were statistically different according to the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
Table 4.
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (p < 0.05). Values with different lowercase superscript letters in each column were statistically significantly different at each time point between the experimental treatment groups based on the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.
Table 5.
Data are presented as means ± standard deviations. Values with different uppercase superscript letters in each row were statistically significantly different for each treatment between the time points before (T0) and after (T1) treatment based on the Tukey test (p < 0.05). Values with different lowercase superscript letters in each column were statistically significantly different at each time point between the experimental treatment groups based on the Tukey test (p < 0.05).
HP, hydrogen peroxide (38% HP; Opalescence Boost, Ultradent Products Inc., South Jordan, UT, USA); CP, carbamide peroxide (10% CP; Opalescence 10% PF, Ultradent Products Inc.); LW, Listerine Whitening (Johnson & Johnson do Brasil Indústria e Comércio de Produtos para Saúde Ltda, São Paulo, SP, Brazil); CPW, Colgate Plax Whitening (Colgate-Palmolive Ind. Ltda., São Paulo, SP, Brazil); CG, control group.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download