Abstract
Purpose
The 1-year outcomes of intravitreal dexamethasone implants in diabetic macular edema (DME) patients were evaluated.
Methods
The medical records of 67 patients (70 eyes) with DME were reviewed retrospectively. All patients were treated with intravitreal dexamethasone implants more than twice a year and followed up for at least 1 year from the first dexamethasone implant injection. The best-corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) were measured at every visit after the first injection. Adverse effects, including cataract formation and elevation of IOP, were analyzed.
Results
The mean patient age was 58.72 ± 9.91 years and 38 patients were male. The average number of injections was 2.73 ± 0.65 and the interval between injections was 21.52 ± 8.47 weeks. The average baseline BCVA and CMT were 0.59 ± 0.30 logMAR and 457.17 ± 133.10 µm, respectively. The BCVA was significantly improved from 3 months after the first injection, but this result did not last for 1 year. The CMT was significantly decreased compared to baseline for 1 year (p < 0.001). Group analysis revealed that the BCVA improved significantly in the group with HbA1c < 8.1% compared to the group with HbA1c ≥ 8.1% (p = 0.044), and the CMT improved significantly in the group with absent subretinal fluid compared to the group with the presence of subretinal fluid (p = 0.009). An elevated IOP was observed in 18 eyes (24%), and cataract formation was observed in 10 eyes (14%).
Figures and Tables
Figure 1
BCVA and CMT results. (A) BCVA was significantly increased at 1, 3, 9 months after the first injection. (B) CMT was decreased at 1, 3, 6, 9, 12 months after the first injection. BCVA = best corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; CMT = central macular thickness; mon = month(s). *p < 0.05, against baseline by paired t-test.
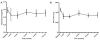
Figure 2
Mean changes in the intraocular pressure. Mean changes in the intraocular pressure was significantly increased at 1, 3, 6, 9, 12 months after the first injection. mon = month(s). *p < 0.05, against baseline by paired t-test.
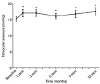
Figure 3
Univariate analysis (by GEE) for BCVA and CMT change from baseline. (A) Pseudophakic eyes showed no significant difference in BCVA and CMT compared to phakic eyes. (B) Vitrectomized eyes showed no significant difference in BCVA but significantly large decrease in CMT compared to non-vitrectomized eyes. (C) Absence of SRF group showed no significant difference in BCVA but significantly large decrease in CMT compared to the presence of SRF group. (D) Focal DME eyes showed significant increase in BCVA but no difference in CMT compared to diffuse DME eyes. (E) HbA1c < 8.1% group showed significant improvement of BCVA as well as decrease of CMT compared to HbA1c ≥ 8.1% group. BCVA = best corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; GEE = generalized estimating equation; mon = month(s); CMT = central macular thickness; SRF = subretinal fluid; DME = diabetic macular edema.
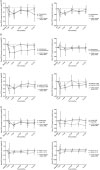
Table 1
Baseline characteristics of enrolled patients

Values are presented as mean ± standard deviation or n (%).
DM = diabetes mellitus; DME = diabetic macular edema; VEGF = vascular endothelial growth factor; BCVA = best corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; CMT = central macular thickness; IOP = intraocular pressure; SRF = subretinal fluid.
Table 2
Adverse effects of ozurdex injection and results on the injection number and the interval during follow-up period

References
1. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–564.


2. Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984; 28:452–461.


3. Photocoagulation for diabetic macular edema. early treatment diabetic retinopathy study report number 1. early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985; 103:1796–1806.

4. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14:223–232.


5. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011; 118:615–625.

6. Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007; 114:1860–1867.



7. Avitabile T, Azzolini C, Bandello F, et al. Aflibercept in the treatment of diabetic macular edema: a review and consensus paper. Eur J Ophthalmol. 2017; 27:627–639.

8. Diabetic Retinopathy Clinical Research Network. Aiello LP, et al. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology. 2011; 118:e5–e14.

9. Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011; 152:686–694.


10. Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61.


11. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–224. discussion 224-5.

12. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134.e3–1146.e3.

13. Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010; 128:289–296.


14. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014; 121:1904–1914.


15. Moon BG, Lee JY, Yu HG, et al. Efficacy and safety of a dexamethasone implant in patients with diabetic macular edema at tertiary centers in Korea. J Ophthalmol. 2016; 2016:9810270.

16. Kim TH, Yoon CK, Lee JE, et al. One-year outcome of intravitreal dexamethasone implant for macular edema secondary to central retinal vein occlusion. J Korean Ophthalmol Soc. 2016; 57:1918–1925.

17. Park DH, Ha SJ, Lee SJ. Intraocular pressure elevation after 0.7 mg intravitreal dexamethasone (Ozurdex®) implantation: a one year follow-up. J Korean Ophthalmol Soc. 2015; 56:891–899.

18. Ozkok A, Saleh OA, Sigford DK, et al. THE OMAR STUDY: Comparison of ozurdex and triamcinolone acetonide for refractory cystoid macular edema in retinal vein occlusion. Retina. 2015; 35:1393–1400.

19. Dang Y, Mu Y, Li L, et al. Comparison of dexamethasone intravitreal implant and intravitreal triamcinolone acetonide for the treatment of pseudophakic cystoid macular edema in diabetic patients. Drug Des Devel Ther. 2014; 8:1441–1449.



20. Dutra Medeiros M, Postorino M, Navarro R, et al. Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica. 2014; 231:141–146.


21. Bansal P, Gupta V, Gupta A, et al. Efficacy of ozurdex implant in recalcitrant diabetic macular edema--a single-center experience. Int Ophthalmol. 2016; 36:207–216.


22. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014; 121:1904–1914.

23. Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013; 120:1843–1851.

24. Scaramuzzi M, Querques G, Spina CL, et al. Repeated intravitreal dexamethasone implant (Ozurdex) for diabetic macular edema. Retina. 2015; 35:1216–1222.


25. Chou TH, Wu PC, Kuo JZ, et al. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye (Lond). 2009; 23:1360–1363.


26. Singh RP, Wykoff CC, Brown DM, et al. Outcomes of diabetic macular edema patients by baseline hemoglobin A1c: analyses from VISTA and VIVID. Ophthalmol Retina. 2017; 1:382–388.

27. Pacella F, Ferraresi AF, Turchetti P, et al. Intravitreal injection of ozurdex (R) implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol Eye Dis. 2016; 8:11–16.


28. Lee H, Kang KE, Chung H, Kim HC. Prognostic factors for functional and anatomic outcomes in patients with diabetic macular edema treated with dexamethasone implant. Korean J Ophthalmol. 2018; 32:116–125.



29. Çevik SG, Yılmaz S, Çevik MT, et al. Comparison of the effect of intravitreal dexamethasone implant in vitrectomized and nonvitrectomized eyes for the treatment of diabetic macular edema. J Ophthalmol. 2018; 2018:1757494.

30. Medeiros MD, Alkabes M, Navarro R, et al. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2014; 30:709–716.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download