Abstract
Purpose
To evaluate the types, frequency, and causes of complications at the donor site after conjunctivo-limbal autograft for primary or recurrent pterygium treatment.
Methods
From January 2010 to December 2016, 91 eyes of 91 patients (male, n = 37; female, n = 54; mean age, 53.29 ± 10.73 years), diagnosed with primary or recurrent pterygium, and who were followed up for 6 months or longer after conjunctivo-limbal autograft, were enrolled in this study retrospectively.
Results
Of the 91 eyes, 27 eyes (29.7%) developed a conjunctival scar on the donor site and 36 eyes (39.6%) had localized vascularization. Eighteen eyes (19.8%) had a conjunctival scar and localized vascularization. Conjunctival granuloma and limbal stem cell deficiency occurred in one eye (1.1%). Multiple regression analysis showed that having a conjunctival scar and localized vascularization were significantly correlated with young age (p < 0.001), but were not significantly correlated with gender, pterygium type, dry eye, conjunctivochalasis, hypertension, diabetes, anticoagulant treatment, graft size, or delayed epithelial-wound healing.
Conclusions
Conjunctival scarring or localized vascularization on the donor site after conjunctivo-limbal autograft for the treatment of the pterygium was found to be significantly higher in younger patients. Therefore, it is recommended that conjunctivo-limbal autograft should be adequately explained for donor-site complications in younger-aged pterygium patients.
Figures and Tables
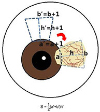 | Figure 1The size of conjunctivolimbal autograft. The width was calculated by the trapezoidal width formula. a = base; b = upper side of base; h= height. |
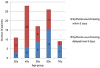 | Figure 2The number of patients according to the age group classified by period of epithelial wound healing. The rate of delayed epithelial wound healing was higher in the older age group. |
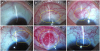 | Figure 3Anterior segment photographs show donor-site complication. No complication (A). Conjunctival scarring (circle) (B). Vascularization (circle) (C). Conjunctival scarring and vascularization (circle) (D). Conjunctival granuloma (circle) (E). Focal lim - bal stem cell deficiency (circle) (F). |
References
1. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001; 119:695–706.

2. Pinkerton OD, Hokama Y, Shigemura LA. Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 1984; 98:225–228.


3. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999; 128:280–287.


4. Lee SH, Jeong HJ. Immune reactions in pterygium. J Korean Ophthalmol Soc. 1987; 28:927–933.
5. Dadeya S, Malik KP, Gullian BP. Pterygium surgery: conjunctival rotation autograft versus conjunctival autograft. Ophthalmic Surg Lasers. 2002; 33:269–274.


6. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–1240.


7. Güler M, Sobaci G, Ilker S, et al. Limbal-conjunctival autograft transplantation in cases with recurrent pterygium. Acta Ophthalmol (Copenh). 1994; 72:721–726.

8. Ti SE, Tseng SC. Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol. 2002; 13:204–212.

9. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001; 108:449–460.


10. Sánchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998; 82:661–665.


11. Polute P, Heilinggenhaus A, Koch J, et al. Long-term results of autologous conjunctival-limbus transplantation in pterygium. Klin Monbl Augenheilkd. 1998; 213:9–14.

12. Shimazaki J, Yang HY, Tsubota K. Limbal autograft transplantation for recurrent and advanced pterygia. Ophthalmic Surg Lasers. 1996; 27:917–923.

13. Kim CH, Lee JK, Park DJ. Recurrence rates of amniotic membrane transplantation, conjunctival autograft and conjunctivolimbal autograft in primary pterygium. J Korean Ophthalmol Soc. 2009; 50:1780–1788.

14. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96:709–723.


15. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002; 109:1752–1755.


16. Vrabec MP, Weisenthal RW, Elsing SH. Subconjunctival fibrosis after conjunctival autograft. Cornea. 1993; 12:181–183.


17. Leonardi A, Radice M, Fregona IA, et al. Histamine effects on conjunctival fibroblasts from patients with vernal conjunctivitis. Exp Eye Res. 1999; 68:739–746.


18. Leonardi A, Cortivo R, Fregona I, et al. Effects of Th2 cytokines on expression of collagen, MMP-1, and TIMP-1 in conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2003; 44:183–189.


19. Zada M, Pattamatta U, White A. Modulation of fibroblasts in conjunctival wound healing. Ophthalmology. 2018; 125:179–192.


20. Tan D. Conjunctival grafting for ocular surface disease. Curr Opin Ophthalmol. 1999; 10:277–281.


21. Jhagta HS, Jain P. Limbal stem cell deficiency: a review. J Ophthalmol Clin Res. 2015; 3:71–75.
22. Holland EJ, Schwartz GS. Iatrogenic limbal stem cell deficiency. Trans Am Ophthalmol Soc. 1997; 95:95–107.


23. Bae SG, Lee JK, Park DJ. Effectiveness of wide excision of subconjuncival fibrovascular tissue with conjunctivo-limbal autograft in pterygium surgery. J Korean Opthalmol Soc. 2012; 53:215–222.
25. Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999; 79:1479–1487.

26. Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001; 117:1027–1035.


27. Keylock KT, Vieira VJ, Wallig MA, et al. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008; 294:R179–R184.

28. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007; 117:1219–1222.







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download