Abstract
Purpose
To investigate the clinical manifestations and properties of remnant particles in the subconjunctival space after high-frequency radio-wave electrosurgery for conjunctivochalasis.
Methods
We performed a retrospective, observational case series with in vitro experimental imaging in nine eyes from eight patients who presented with small dark-gray lesions during follow-up after high-frequency radio-wave electrosurgery for conjunctivochalasis. General examination including slit-lamp examination and visual acuity testing was performed preoperatively and postoperatively. During follow-up, we evaluated remnant particles and any other complications including granuloma or conjunctival injection with slit-lamp photography and anterior optical coherence tomography. Coagulation tips were investigated with scanning electron microscope and energy dispersive X-ray spectroscopy to analyze the insulating electrode and assess changes to tips after repeated use.
Results
None of the patients included in this study experienced any change in visual acuity or major complications postoperatively. Small dark-gray lesions (0.3 to 0.5 mm in size) were observed in the inferior bulbar sub-conjunctival space in the location where high-frequency radio-wave electrosurgery had been performed. Cirrus high-definition optical coherence tomography images revealed focal hyper-reflection with a posterior shadow, suggesting foreign particles. Scanning electron microscopy and energy dispersive X-ray spectroscopy imaging analysis revealed peaks of carbon and fluorine complexes, consistent with the polytetrafluoroethylene coating on the electrode.
Conclusions
There were no instances of inflammatory reaction, particle migration, or major complications due to particles. Physicians should be aware of the possibility of remnant polytetrafluoroethylene particles in subconjunctival tissue when using insulated coagulation tips subjected to repeat sterilization.
Conjunctivochalasis is the presence of loose redundant conjunctival tissue in bulbar conjunctiva and is responsible for several ocular signs and symptoms such as decreased vision, discomfort, pain, dryness injections, and subconjunctival hemorrhage [123]. Treatment options for conjunctivochalasis include topical lubrication and steroids [1], simple resection of redundant tissue [2456], electrocoagulation, and high-frequency radio-wave electrosurgery (HRES) [7891011].
Following our initial description of a simple surgical approach with HRES to treat conjunctivochalasis [11], the procedure has been expanded the treatment of lymphangiectasis of the conjunctiva [8], persistent chemosis after blepharoplasty [10], superior limbic keratoconjunctivitis [7], and conjunctival cyst removal [9]. However, during the follow-up period after HRES, we identified small remnant particles in the subconjunctival area in some patients. The purpose of this study was to report potential issues related to remnant polytetrafluoroethylene (PTFE) particles that may detach from the electrode coating of the needle, resulting in complications such as inflammation. To the best of our knowledge, this is the first study to report the presence of PTFE particles in the subconjunctival area after HRES.
This was a retrospective, observational case series of the medical records of patients who underwent HRES in the outpatient clinic of Kangbuk Samsung Hospital in Seoul, Korea. The inclusion criteria before initiating medical record review were 1) a diagnosis of conjunctivochalasis and 2) treatment with HRES in the previous 36 months with at least three months of available follow-up. Patients for whom the diagnosis of conjunctivochalasis was unclear and those who underwent conjunctival surgery and showed any findings of previous conjunctival pyogenic infection or inflammation before HRES were excluded. Collected data from medical charts and available anterior segment photographs were patient demographic information, previous medications, and grade of conjunctivochalasis. For grading of conjunctivochalasis, we used the lid-parallel conjunctival folds classification. Briefly, conjunctivochalasis was divided into four stages of (0) no conjunctival fold, (I) one small fold, (II) greater than two folds but not higher than the tear meniscus, and (III) multiple folds higher than the tear meniscus. To protect patient and physician confidentiality, all data were de-identified at the time of collection and reported in aggregate. No personal health information was collected. This study adhered to the principles outlined in the Declaration of Helsinki for human research, and all participants provided written informed consent. The study protocol was approved by the institutional review board of Kangbuk Samsung Hospital (2018-03-011).
Symptomatic relaxation of the redundant conjunctiva in the inferior bulbar conjunctiva was noted in all patients who underwent HRES. All procedures were performed by the same surgeon (CYC) under topical anesthesia (0.5% proparacaine) as previously described [7891011]. HRES was performed in approximately 10 to 15 locations in each bulbar area according to disease severity using an insulation-coated fine-needle electrode (Ellman Surgitron; Ellman International, Hewlett, NY, USA). All patients were postoperatively instilled and treated with topical antibiotic and steroid eyedrops (0.5% levofloxacin and 0.1% fluorometholone; Santen, Osaka, Japan) for 3 weeks.
During the follow-up period after HRES, we evaluated whether there was inflammation on the conjunctiva or foreign material present after surgery and noted any changes in shape or location of foreign bodies on slit-lamp examination and photography. We did not consider subconjunctival hemorrhage or generalized injection that occurred after the procedure to be an inflammatory effect associated with particles. However, presence of localized injection or granuloma formation around a particle was considered a particle-associated side effect. To evaluate the small, dark-gray foreign material in the subconjunctival tissue during the follow-up period, we performed cirrus high-definition optical coherence tomography (HD-OCT; Carl Zeiss Meditec, Dublin, CA, USA). To evaluate changes in reused coagulation tips, we performed imaging analysis of peeling degree of the insulated components.
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) were used for imaging analysis of remnant particles from the coagulation tips after surgery.
Electrodes (new, used once, or used three times) were mounted and sputtered with gold before being examined via high-resolution scanning electron microscope (Quanta Inspect F; FEI Co., Hillsboro, OR, USA) with a spatial resolution of less than 1 nm. Secondary electrons were probed to obtain SEM images at a typical acceleration voltage of 10 kV.
Element analysis is a technique used to analyze the chemical makeup of a sample. To enable qualitative and quantitative microanalyses, an energy-dispersive X-ray spectrometer (silicon drift detector; Oxford Instruments, Oxford, UK) was attached to the SEM. As a type of spectroscopy, this approach investigates a sample based on interactions between electromagnetic radiation and matter and analyzes X-rays emitted by that matter in response to bombardment with charged particles.
A total of nine eyes (4.2%) of eight patients from a total of 212 eyes seen over the preceding three years were identified as having dark-gray lesions in the subconjunctival space during the follow-up period on slit-lamp examination (Table 1). Patient age ranged from 55 to 72 years, with a mean of 61 years. There were no complications during the procedures except subconjunctival hemorrhage in six cases. All other routine ophthalmic examinations were within the normal range. Conjunctivochalasis was evaluated according to the lid-parallel conjunctival folds classification system. Using this system, 11 eyes were categorized as stage II conjunctivochalasis, and four eyes were categorized as stage III. Symptoms resolved in all patients with stage II conjunctivochalasis and were improved significantly for those with stage III conjunctivochalasis with respect to duration and intensity. All patients reported satisfaction with the results. No major complications were detected during the follow-up period.
All the lesions (0.3 to 0.5 mm in size) from patients at the inferior bulbar conjunctiva were located at the site of HRES (Fig. 1, 2, 3). Cirrus HD-OCT revealed focal hyper-reflection signals with posterior shadowing in subconjunctival tissue different from the surrounding structures, blood vessels, and melanin pigment, suggesting the presence of foreign particles (Fig. 4). No changes in shape or location of particles were noted during the follow-up period.
SEM imaging showed that new electrodes had a well-wrapped PTFE and a smooth and regularly coated surface (Fig. 5A, 5B). However, after use of the device for HRES, we observed several peeled-off particulates (Fig. 5C, 5D). In addition, isolated PTFE particles that had been removed from the shaft were observed (Fig. 5E, 5F).
We next performed scanning SEM/EDS, focusing on the area where the PTFE coatings were suspected to be located on the insulated shaft and the tip of the electrode. The results showed that the part of the electrode without electrical insulation (Fig. 6A) was composed of wolframium (tungsten) (Fig. 6B); however, in the portion of the shaft with insulation (Fig. 6C), we identified an increased peak for fluorine at 0.68 keV (Fig. 6D). The high compositions of carbon and fluorine were attributed to the PTFE coating on the shaft. EDS revealed that the part without insulation contained 93.12% wolframium, 4.11% carbon, and 02.77% oxygen, while the insulated part of the electrode contained 36.45% wolframium, 29.60% fluorine, 26.85% carbon, and 7.09% oxygen (Fig. 6B, 6D).
Conjunctivochalasis has traditionally been treated using eyedrops and surgery during which sutures are used to rejoin margins after conjunctival resection [1]. One surgical technique consists of creating a simple crescent excision of the inferior bulbar conjunctiva [1]. The HRES system minimizes postoperative discomfort and promotes fast healing without suture problems in ophthalmic disease. HRES is a quick, safe, and effective surgical option to resolve various diseases of the ocular surface. Indeed, the indications for HRES include conjunctivochalasis [1112], lymphangiectasis of the conjunctiva [8], persistent chemosis after blepharoplasty [10], superior limbic keratoconjunctivitis [7], and removal of conjunctival cysts [9].
Institution- and department-specific differences exist for electrocautery surgical procedures in ophthalmic fields for conjunctival surgery and other general surgical conditions. In conjunctival surgeries, a sharp, fine tip is used to penetrate the conjunctival tissue, and coagulation is performed within the closed tissue. Conversely, non-ophthalmic procedures typically utilize the surface of the wound to dissect tissue and/or control bleeding. Penetration of the coagulation tip into tissue is more likely to promote resistance to the insulation-coated portion, causing PTFE to dissociate from the tip shaft and become enclosed within the tissue.
We hypothesized several mechanisms by which PTFE-wrapped devices may lose PTFE. First, repeated heating and high-frequency radio waves may render the coating unstable. With very little additional force, the coating material could be weakened and peeled off. Second, during repeated penetration of the conjunctival tissue, the surface resistance of the shaft may alter the stability of the insulation coating. Lastly, the sterilization process necessary to reuse the electrodes could affect the stability of the surface structure of the tip. Following damage by these mechanisms, it is possible that the PTFE coating be removed from particles at the surgical site.
PTFE is a fluorocarbon solid and is considered nonreactive due to the strength of carbon-fluorine bonds. In animal experiments, PTFE has been linked to a wide range of health concerns, including liver toxicity [1314]; mortality [1314]; decreased body weight [1314]; developmental delays [13]; behavioral changes [15]; abnormal mammary-gland development [16]; and changes in hormone levels (e.g., estrogen, testosterone, and decreased thyroid hormones) [131417], cholesterol levels [181920], the immune system [212223], and cell cycle [2425], among other effects [262728]. HRES is performed at a relatively low temperature where PTFE is considered stable and nontoxic [29]. However, no studies to date have evaluated the safety of retained PTFE particles [303132], including in the subconjunctival surgical site. In our study, HD-OCT images revealed focal hyper-reflection signals with posterior shadowing in the subconjunctival area. These signals did not appear to be natural conjunctival structures such as blood vessels or lymphatics. Thus, the pathologies observed in these patients were attributed instead to particles of foreign material.
The limitations of this study were the relatively short follow-up period, small sample size, and retrospective study design. As a result of these limitations, we were unable to assess preoperative anterior photography. A larger series with long-term follow-up is needed to confirm the incidence rate of remnant particles in HRES for conjunctivochalasis and to establish whether PTFE undergoes widespread distribution throughout the body or affects other organ systems. In one case in our study, the remnant particles were located near the conjunctival vessels and absorbed through lymphatic channels, which could theoretically cause systemic side effects. Overall, we found that the incidence of remnant particles was approximately 4% to 5% of 200 cases. As PTFE has a half-life of 4 to 5 years, the possibility of systemic side effects of PTFE should be taken seriously. In addition, if any changes to the lesion site are observed, additional surgical resection may be needed.
In summary, some patients may present with subconjunctival remnant particles of PTFE following HRES. No inflammatory reactions were observed among these patients. However, we should remain aware of the possibility of remnant PTFE particles in subconjunctival tissue and related complications, including migration of particles and other systemic side effects.
Figures and Tables
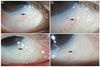 | Fig. 1Slit-lamp photographs of a dark-gray lesion at the inferior bulbar conjunctiva (arrow) observed in a 63-year-old woman. No observed changes were observed in the lesion 3 months after surgery: (A,B) 1 month postoperatively and (C,D) 3 months postoperatively. |
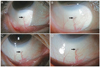 | Fig. 2Slit-lamp photographs of a dark-gray lesion at the inferior bulbar conjunctiva (arrow) observed in a 67-year-old woman. No observed changes were observed in the lesion 3 months after surgery: (A,B) 1 month postoperatively; (C,D) 3 months postoperatively. |
 | Fig. 3Slit-lamp photographs of a 57-year-old man. One month after surgery, several tiny dark-gray lesions at nasal and temporal conjunctiva (arrow) were observed: 1 month postoperatively (A) at the nasal conjunctiva and (B) at the temporal conjunctiva. |
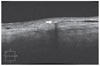 | Fig. 4High-definition optical coherence tomography images of a 63-year-old woman (right eye). Three months postoperatively, high-definition optical coherence tomography image showed a focal hyper-reflection with posterior shadowing (arrow) in the subconjunctival area. |
 | Fig. 5Scanning electron microscopy images of the shaft of an insulated tip. (A,B) A new insulated fine-needle electrode. (C,D) A partially damaged insulated surface after a single use. (E,F) A severely damaged insulated surface after multiple uses. A large polytetrafluoroethylene particle peeling away from the shaft was noted (arrow) (scanning electron microscope, ×200, ×500). |
 | Fig. 6Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy spectrum (EDS) images of the shaft of an insulated tip: (A) SEM image (×1,000) of the tip without insulation. (B) EDS image of the tip showing a prominent peak for wolframium (W) as well as other associated elements, including carbon (C) and oxygen (O). (C) SEM image (×1,000) of the insulated shaft. (D) EDS image of the shaft showing a prominent peak for fluorine (F) as well as for other associated elements, including C, O, and W. W = wolframium; C = carbon; O = oxygen; F = fluorine; Wt% = weight percent; At% = atomic percent; ZAF = ZAF corrections. |
References
2. Liu D. Conjunctivochalasis: a cause of tearing and its management. Ophthalmic Plast Reconstr Surg. 1986; 2:25–28.

3. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998; 43:225–232.

5. Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1beta and tumor necrosis factor-alpha. Invest Ophthalmol Vis Sci. 2000; 41:2922–2929.

6. Otaka I, Kyu N. A new surgical technique for management of conjunctivochalasis. Am J Ophthalmol. 2000; 129:385–387.


7. Ahn JM, Choi CY, Seo KY. Surgical approach with high-frequency radiowave electrosurgery for superior limbic keratoconjunctivitis. Cornea. 2014; 33:210–214.


8. Han KE, Choi CY, Seo KY. Removal of lymphangiectasis using high-frequency radio wave electrosurgery. Cornea. 2013; 32:547–549.


9. Park J, Lee S, Suh E. Removal of conjunctival cyst with high-frequency radio-wave electrosurgery. Can J Ophthalmol. 2015; 50:378–383.


10. Woo KI, Choi CY. High-frequency radiowave electrosurgery for persistent conjunctival chemosis following cosmetic blepharoplasty. Plast Reconstr Surg. 2014; 133:1336–1342.


11. Youm DJ, Kim JM, Choi CY. Simple surgical approach with high-frequency radio-wave electrosurgery for conjunctivochalasis. Ophthalmology. 2010; 117:2129–2133.


12. Suh JS, Choi S. The effect of conjunctivochalasis surgery using a high-frequency radio-wave electrosurgical unit. J Korean Ophthalmol Soc. 2012; 53:1571–1576.

13. Lau C, Thibodeaux JR, Hanson RG, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003; 74:382–392.


14. Seacat AM, Thomford PJ, Hansen KJ, et al. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol Sci. 2002; 68:249–264.


15. Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008; 29:160–169.


16. White SS, Kato K, Jia LT, et al. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol. 2009; 27:289–298.


17. Bookstaff RC, Moore RW, Ingall GB, Peterson RE. Androgenic deficiency in male rats treated with perfluorodecanoic acid. Toxicol Appl Pharmacol. 1990; 104:322–333.


18. Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010; 118:197–202.


19. Sakr CJ, Kreckmann KH, Green JW, et al. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med. 2007; 49:1086–1096.

20. Steenland K, Tinker S, Frisbee S, et al. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009; 170:1268–1278.


21. Fairley KJ, Purdy R, Kearns S, et al. Exposure to the immunosuppressant, perf luorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol Sci. 2007; 97:375–383.

22. Yang Q, Xie Y, Alexson SE, et al. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol. 2002; 63:1893–1900.


23. Yang Q, Xie Y, Eriksson AM, et al. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem Pharmacol. 2001; 62:1133–1140.

24. Alexander BH, Olsen GW, Burris JM, et al. Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup Environ Med. 2003; 60:722–729.



25. Gilliland FD, Mandel JS. Mortality among employees of a perf luorooctanoic acid production plant. J Occup Med. 1993; 35:950–954.

26. Bloom MS, Kannan K, Spliethoff HM, et al. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol Behav. 2010; 99:240–245.


27. Dallaire R, Dewailly E, Pereg D, et al. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009; 117:1380–1386.



28. Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health. 2007; 81:231–246.


30. Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007; 115:1670–1676.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download