This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To investigate the effects of transplantation of a circumferentially-trephined autologous oral mucosal graft using a vacuum trephine on ocular surface reconstruction in patients with limbal stem cell deficiency.
Methods
Patients with a limbal stem cell deficiency who underwent transplantation of autologous oral mucosal graft performed by a particular surgeon in Seoul National University Hospital were included. The medical records of these five patients were retrospectively reviewed. The lower labial mucosal graft inside the inferior lip was trephined to a depth of 250 µm using a donor vacuum trephine with a 9-mm diameter. Outside markings were made using a 14-mm intraoperative keratometer. The oral mucosal graft was dissected under a microscope using a Beaver mini-blade as either a ring or a crescent-shaped strip with a 5-mm width. The mucosal graft was transplanted onto the limbus in the limbal-deficient eye. Best-corrected visual acuity and corneal status were measured during the follow-up period.
Results
Four patients were diagnosed with Stevens-Johnson syndrome and one was diagnosed with atopy-associated immune keratitis. The mean follow-up period was 10.4 ± 2.9 months. After 4 months, visual acuity improved in all patients, and the mean improvement in logarithm of the minimum angle of resolution visual acuity was 0.526 ± 0.470 (range, 0.15 to 1.10). Corneal surface erosion and neovascularization decreased in four patients, and stromal opacity decreased in two patients. The engraftments maintained ocular surface stabilization in four of the five patients at the last follow-up.
Conclusions
Transplantation of circumferential autologous oral mucosal grafts may be effective for the treatment of limbal stem cell deficiency.
Keywords: Reconstructive surgical procedures, Stevens-Johnson syndrome, Transplants
The cornea is stabilized by well-functioning stem cells that reside in the basal layer of the limbus, the transitional zone between the corneal conjunctiva and the bulbar conjunctiva [
1]. When the limbus suffers limited damage, the circumferential migration of stem cells from the adjacent limbus maintains limbal integrity. However, when the limbus suffers damage so severe that this mechanism becomes ineffective, sources of corneal epithelial cells are depleted, leading to signs of various disorders, such as conjunctival ingrowth into the cornea, superficial neovascularization, recurrent corneal erosions, or corneal opacities, and resulting in severe vision loss [
2]. These pathologic conditions are considered limbal stem cell deficiencies (LSCD), which are caused by external injuries (such as chemical or thermal burns or recurrent microbiological infections) or ocular diseases (such as ocular cicatricial pemphigoid, Stevens-Johnson syndrome [SJS], and aniridia) [
3].
The first method developed to treat LSCD was limbal autograft transplantation harvested from the contralateral healthy eye [
4]; however, this strategy can be attempted only in unilaterally involved LSCD, and it increases the risk of LSCD in the contralateral eye due to the large excision of the limbus [
4]. Limbal allograft transplantation is now a standard treatment for bilateral LSCD. However, graft rejection is common, so the procedure requires long-term use of systemic immunosuppression, which has the potential for systemic adverse reactions [
567]. Particularly in cases of SJS, which is a common cause of LSCD, the rejection rate of limbal allograft transplantation is higher than that of other indications [
8].
To avoid these complications, transplantation of cultivated autologous or allo-limbal epithelial cells [
59101112] or transplantation of cultivated autologous mucosal epithelial cells [
131415] have been performed in bilateral total LSCD. Several studies have also reported favorable results from autologous oral mucosal epithelial transplantations for ocular surface reconstruction in LSCD [
13141516]. We previously performed a clinical trial on corneal reconstruction using a cultivated oral mucosal sheet in bilateral LSCD, which showed a favorable outcome [
17]. However, due to limited resources and the high cost of making a cell sheet in Korea, we attempted an alternative, simpler method for treating LSCD through direct transplantation of a circumferentially-trephined oral mucosal graft for ocular surface reconstruction. In this study, we retrospectively report the surgical outcomes of five patients who were diagnosed with partial or total LSCD and received autologous circumferentially-trephined oral mucosal graft transplantation.
Materials and Methods
Subjects
The protocol of this study adhered to the tenets of the Declaration of Helsinki and was approved by review and ethics board in Seoul National University Hospital (H-1803-118-932). The medical records of patients who were diagnosed with partial or total LSCD at the corneal clinic in Seoul National University Hospital and who received oral mucosal graft transplantation onto the limbus from May 1, 2015, to April 30, 2017, were reviewed. The diagnosis of LSCD was based on clinical findings including loss of the limbal palisades of Vogt, an irregular corneal epithelium, superficial corneal vascularization, conjunctival ingrowth, and persistent epithelial defect following a history of ocular-surface disease or external injury [
18]. The surgery was performed in patients who could not receive limbal allograft transplantation because of their general health or who did not respond to amniotic membrane transplantation (AMT) or medical treatment. Cases with a follow-up duration of more than 4 months were included. Tectonic oral mucosal graft transplantations for corneal perforation were excluded, as were the cases of patients who had undergone keratolimbal allograft transplantation or penetrating keratoplasty before mucosal grafting. Demographic factors regarding age, sex, best-corrected visual acuity (BCVA), etiology of LSCD, and prior surgeries were collected and are shown in
Table 1. Risk factors for LSCD, such as chronic inflammation, symblepharon, and lid deformity, were also collected and are also shown in
Table 1. The severity of ocular inflammation was graded as either mild (+), moderate (++), or severe (+++), as outlined in a previous report [
19].
Surgical procedures
After obtaining written informed consent from each patient regarding the surgical procedure as well as relevant alternatives, risks, benefits, and publication, all surgeries were performed under general anesthesia by a single surgeon (MKK).
The oral labial mucosal graft was harvested from inside the inferior lip (
Fig. 1A). Two-percent lidocaine mixed with epinephrine (1 : 100,000) was injected submucosally to create a submucosal space for dissection. Markings for the inside incision were made with a donor vacuum trephine (Hessburg-Barron trephine; Katena Products, Denville, NJ, USA) with a 9-mm diameter using gentian violet ink, and outside markings were made using a 14-mm intraoperative keratometer (K3-7888, Katena Products) (
Fig. 1A). The oral mucosal graft was dissected under a microscope using either a Beaver mini-blade (Beaver-Visitec International, Waltham, MA, USA) or a Vanna scissor in the form of a ring or crescent-shaped strip with a width of 5 mm and a depth of 250 µm (
Fig. 1B). During these procedures, the mucosal surface was covered with Viscoat (Alcon, Fort Worth, TX, USA) to protect it from drying. Harvested mucosae were submerged in 5% povidone iodine for one minute and subsequently irrigated with BSS solution (Alcon) mixed with gentamicin (1 mg/mL).
Prior to graft transplantation, the fibrotic vascular membrane and the pannus overlying the cornea were fully removed (
Fig. 1C), and a conjunctival peritomy was circumferentially performed along the limbal stem cell-deficient area (
Fig. 1D). In eyes with a symblepharon, conjunctival dissection was performed to release the conjunctival adhesion, and an amniotic membrane was permanently transplanted to cover the conjunctival defect area.
When the corneal surface was smooth, the mucosal graft was directly transplanted (cases 2 to 5). When the corneal surface was irregular and not smooth, an amniotic membrane was permanently transplanted onto the cornea before mucosal grafting (case 1). The free mucosal graft was placed onto the limbus, and the inside margin of the graft was fixed with 10-0 nylon anchoring sutures (
Fig. 1E, 1F). Tissue glue (TISSEEL; Baxter Healthcare Corp, Deerfield, IL, USA) was used to firmly attach the mucosal graft to the bed of the recipient limbal area (
Fig. 1G). After trimming excess tissue in the outside margin of the graft, it was sutured to the conjunctiva with continuous 10-0 nylon sutures to hold the graft onto the limbus (
Fig. 1H). Transient AMT using a manufactured amniotic membrane (SK Bioland, Cheonan, Korea) was performed using 10-0 nylon sutures to protect the transplanted graft during the healing process (
Fig. 1I).
Postoperative care
After surgery, a bandage contact lens (Acuvue Oasys with hydraclear plus; Johnson & Johnson, Jacksonville, FL, USA) was used to protect the wound until all epithelial defects of the cornea were healed. Over the month following surgery, all patients received either topical 0.5% moxifloxacin hydrochloride (Vigamox, Alcon) or 0.3% gatifloxacin hydrochloride (Gatiflox; Handok, Seoul, Korea) four times a day, along with 0.5% loteprednol suspension (Lotemax; Bausch & Lomb, Tampa, FL, USA) or 0.1% fluorometholone acetate solution (Flarex, Alcon) on the same schedule, and autologous serum eye drops (prepared in the institutional blood bank as described in the literature)2019-02-11 [
20] every 2 hours. Thereafter, topical steroid- and autologous serum-use were tapered off based on the status of the ocular surface. The first week following surgery, patients were prescribed 30 mg of oral prednisolone a day, which was tapered off to 20 mg a day for the second week and 10 mg a day for the third week. Patients were additionally encouraged to gargle with 5% chlorohexidine solution four times a day to aid the healing of the oral mucosa. Preservative-free artificial tears were also prescribed.
Outcome measures
The primary outcome measured was BCVA, and the secondary outcomes were corneal status including the staining score, neovascularization, and corneal opacity. These results were compared to preoperative measurements. Corneal staining was graded in each of five corneal zones using the NEI grid (superior, nasal, central, inferior, and temporal) with scores of 0 to 3 (0 = normal, 1 = mild, 2 = moderate, 3 = severe) [
21]. Corneal opacity was graded on a scale of 0 to 4 (0 = clear cornea, 1 = mild corneal edema visible only on slit lamp examination, 2 = corneal haze mild enough where one can visualize the iris characteristics, 3 = moderate corneal haze where one cannot visualize the iris characteristics, 4 = severe corneal haze or opacification so that the anterior chamber is not visible) [
14]. Neovascularization was graded on a scale of 1 to 4 (1 = limited to the periphery, 2 = extending to the mid-periphery, 3 = involving the entire cornea, 4 = massively involving the entire cornea) [
22]. Outcomes were measured at 4 months after surgery, and the final engraftments were evaluated during the final examination.
Results
Five eyes from five patients (two males a nd three females) were analyzed (
Table 1). The mean age at operation was 60.6 ± 6.3 years (range, 55 to 67 years) and the mean length of the postoperative follow-up period was 10.4 ± 2.9 months (range, 8 to 16 months). The etiology of LSCD was SJS in four patients and atopy-associated immune keratitis in one patient. Two patients presented with total LSCD (cases 1 and 2) in conjunction with more severe, chronic, grade 3 inflammation, and the other patients presented with partial LSCD. In addition to LSCD, trichiasis was found in four eyes (cases 1, 2, 3, and 5), and symblepharon was found in three eyes (cases 2, 3, and 5). Three patients (cases 2, 3, and 5) had received transient AMT in the acute phase of SJS due to persistent epithelial defects, and one patient (case 2) had undergone entropion repair in the lower lid (
Table 1).
All patients presented with severe vision loss (from HM at 1 foot to 20 / 100) during the preoperative examination. Oral mucosal grafts were successfully transplanted in all eyes without intraoperative complication (
Fig. 2).
After 4 months, visual acuity was improved in all patients, and the mean improvement in visual acuity was 0.526 ± 0.470 logarithm of the minimum angle of resolution (logMAR; range, 0.15 to 1.10 logMAR) (
Fig. 3). Corneal surface erosion disappeared in three patients (cases 2, 3, and 4) and decreased in one patient (case 5). All patients except case 3 showed fully regressed or decreased limbal neovascularization, but the vascularity of the mucosal graft in cases 3, 4, and 5 appeared to increase. Stromal opacity was decreased in two eyes and maintained in two eyes (
Table 2) [
23].
In case 1, which showed increased corneal opacity after the operation, stromal dimpling developed after pannus removal during the operation, and a permanent AMT was performed prior to oral mucosal graft transplantation to fill the space. This resulted in increased corneal haziness blocking the visual axis after the operation. Eleven months later, the stable corneal surface implied graft survival, and penetrating keratoplasty (PKP) with phacoemulsification and intraocular lens implantation was performed. BCVA improved to 20 / 100 at 4 months following this subsequent surgery (
Fig. 4).
The engraftments were successful in four of the five patients (80%) over the entire follow-up period. In case 3, central macular edema developed along with a recurrence of herpes endotheliitis. The macular edema was subsided after injection of both intravitreal bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) and a dexamethasone implant (Ozurdex; Allergan, Irvine, CA, USA). Herpes endotheliitis was managed with oral valaciclovir and topical ganciclovir gel 0.15% (Virgan; Laboratoires Thöa, Clermont-Ferrand, France). Eleven months after the oral mucosal graft transplantation, permanent AMT using tissue glue combined with temporary AMT was performed to prevent progression of the corneal melting. The final BCVA 4 months after the subsequent surgery was count fingers (CF) at one foot, and an additional PKP was to be considered when the herpes endotheliitis was resolved. In case 5, the surface appeared stable at 2 months after the surgery, and use of the therapeutic contact lens was discontinued. At 8 months, visual acuity had decreased to 20 / 200 with a recurrence of surface inflammation showing partial neovascular invasion of the superior corneal surface and limbal injection. Thick fibrosis in the upper tarsal may have induced chronic friction, which resulted in the recurrence of the inflammation. To reduce inflammation, 0.3% gatifloxacin hydrochloride eye drops, 0.1% fluorometholone acetate solution, and a therapeutic contact lens were used.
Discussion
These results show that direct oral mucosal graft transplantations onto the limbus in cases of partial or total LSCD were successful in the reconstruction of the corneal surface and improved vision markedly. In four of the five patients, the grafts were well-maintained and LSCD was successfully treated during the entire follow-up period. The key factors for grafting success were graft thickness (250 µm) and circumferential shape for matching the curved limbal area. We prepared crescent- or ring-shaped mucosal grafts as thin as the depth of a vacuum trephine using microscope-assisted procedures. The split thickness of a mucosal graft is usually determined using a Castroviejo mucotome or a Silvers skin graft knife; however, these instruments were not available in Korea at the time. Instead, we used a donor trephine to harvest the circumferential-shaped oral mucosal graft. The advantages of trephination are that the shape can be made as circular or as crescent-shaped as desired with an easily-controlled diameter and uniform depth. The use of a thin mucosal graft without attachment to the submucosal fat tissue may have contributed to the high success rate of engraftment, along with the rapid wound healing of the harvested labial area. Circular-shaped grafts align well to the circular limbal area without any of the fish-mouth effects found in rectangular grafts.
Oral mucosal grafts have been used for reconstruction of various lid abnormalities including cicatricial entropion, trichiasis, epidermalization, and symblepharon in SJS [
2425]. The direct transplantation of an autologous oral mucosal graft onto the ocular surface in humans for the purpose of treating corneal ulcers or corneal perforations was first reported in 1992 [
26]. In that report, transplantation was performed to maintain the ocular surface, and visual improvement was not achievable because the graft contained submucosal epithelial tissue [
26]. Thereafter, the oral mucosa became a candidate for ocular surface reconstruction. Based on the successful outcomes of transplantation of autologous oral mucosal epithelial cells in a rabbit model [
27], Nakamura and Kinoshita [
28] performed the first ocular surface reconstruction using cultivated autologous oral mucosal epithelial cells. The oral mucosal epithelium expresses keratin 3, which is also expressed by the corneal epithelium. Additionally, progenitor cells with the potential to differentiate into non-keratinized stratified epithelium mimicking the corneal epithelium are contained in the autologous mucosal cells [
282930]. Since the above-mentioned study by Nakamura and Kinoshita [
28], the efficacy and safety of oral mucosal epithelial cell grafts cultivated
ex vivo have been proven both experimentally and clinically [
1314172231].
Our study began with the question of whether circumferential-shaped oral mucosal grafts could survive when directly transplanted onto the limbus. The cost of cultivation is expensive, so oral mucosal epithelial cell grafts cultivated
ex vivo can only be attempted for a limited number of patients in a limited number of settings. Therefore, we tried to perform a simple procedure similar to a SOMET (simple oral mucosal epithelial transplantation) [
13] in the same way that Sangwan developed simple limbal epithelial transplantation to replace cultivated limbal epithelial transplantation [
18]. Additionally, given that the oral mucosa is more easily accessible than a keratolimbal allograft and that the procedure is simpler than mucosal epithelial cell cultivation, an autologous oral mucosal graft should be considered for patients with partial LSCD, not just total LSCD, before their vision is deteriorated.
Directly transplanting autologous oral mucosal grafts for the treatment of LSCD was first attempted in 2011 by Liu et al. [
32]. That study found that the eyes of all seven patients who received oral mucosal graft transplantation for total LSCD showed vision improvement, and the eyes of five patients exhibited peripheral corneal vascularization. One eye developed partial LSCD due to exposure and was re-operated upon. That study also included patients who had previously received several types of other graft transplantations (keratolimbal allograft, lamellar keratoplasty, PKP, AMT and
ex vivo expansion of limbal stem cells) that each eventually failed. Oral mucosal graft transplantation was proposed as an option for cases where various other surgical treatments had previously failed, and it lead to vision improvement compared to pre-surgery values. However, the final visual acuity achieved was relatively poor (from CF at 1 foot to 20 / 70). For that reason, it has not been widely used as a first-line treatment for LSCD, and no other study on direct mucosal graft transplantation has been reported, to the best of our knowledge. Our study was consistent with the previous report by showing improvement in visual acuity and an 80% success rate for engraftment, which support the feasibility of direct mucosal grafting for LSCD.
We not only included patients with total LSCD but also those with partial LSCD, and none of the patients had received any other type of allograft or autograft transplantation aside from transient AMT. The mean visual improvement was 0.526 ± 0.470 logMAR, which was better than the prior report [
32]. This difference in visual outcome resulted from not only differences in patient characters but also differences in surgical techniques. In the previous report, they used a super-blade and scissors to dissect the mucosal graft, but we used vacuum trephine, which allowed us to make very thin mucosal grafts not containing submucosal fat tissue. Cases 4 and 5, which were patients with partial LSCD and mild vision loss, showed good visual acuities of 20 / 67 and 20 / 25, respectively. In cases 2, 3, and 5, where surface erosion or corneal conjunctivalization persisted after AMT, neovascularization decreased, and a smooth corneal surface was obtained after oral mucosal graft transplantation. Additionally, considering that performing a PKP simultaneously with a keratolimbal allograft may be associated with a less favorable outcome [
83334], the oral mucosal graft in case 1 functioned as a successful first step to treat the LSCD and maintain the corneal surface before the PKP was performed. In that case, visual acuity was improved from CF at 1 foot to 0.7 logMAR at 5 months after the subsequent surgery. Increased vascularity around the mucosal graft was found in the partial LSCD patients, consistent with previous reports [
32]. This appeared to be due to a limitation of the mucosal graft itself, which contains prominent vasculature, rather than a sign of graft rejection.
In ocular surface reconstruction, the success rate decreases with increasing conjunctival inflammation and the lack of a healthy and stable tear film, which is contributed to by lid abnormalities such as lagophthalmos, trichiasis, and symblepharon [
2333]. In our study, three patients showed mild symblepharon that was corrected using symblepharolysis and AMT, ultimately resulting in good survival of the graft. For a good success rate of mucosal grafting, careful selection of surgical candidates without a severe symblepharon may be a critical factor. During the follow-up, we found that supportive treatment is critical for maintaining graft health, especially in cases such as tarsal fibrosis that can directly inflict mechanical damage on the graft as well as lead to inflammation as in case 5. If necessary, a therapeutic contact lens or scleral lens should be applied to protect the transplanted graft.
Our study is notable in that it is the first report to present a new technique in the transplantation of circumferential-shaped autologous oral mucosal grafts in LSCD. In addition, it supports a previous report by showing that direct mucosal grafting may be a viable alternative when a keratolimbal allograft or cultivated mucosal epithelial cell transplantation is not available. This study had a limited sample size and a short follow up duration. In our experience, the survival of mucosal grafts is approximately one year, but more studies are needed to confirm that conclusion. In addition, this study is limited in that it is a retrospective review; therefore, we could not obtain adequate information regarding the effects of tear film quality on surgical outcomes. A large, prospective, randomized clinical trial is required to evaluate the long-term outcomes of autologous oral mucosal graft in LSCD.
Figures and Tables
Fig. 1
Key surgical steps of autologous oral mucosal graft transplantation in patients with limbal stem cell deficiency. (A) Markings for inside incisions were made with a 9-mm-diameter donor vacuum trephine using gentian violet ink, and outside markings were made with a 14-mm intraoperative keratometer. (B) The oral mucosal graft was dissected using a Beaver mini-blade or Vanna scissors in a ring or a crescent-shaped strip of 5-mm width and 250-µm depth. (C) Prior to graft transplantation, the fibrotic vascular membrane and pannus overlying the cornea were fully removed and (D) a conjunctival peritomy was circumferentially performed along the limbal stem cell-deficient area. (E) The free mucosal graft was placed onto the limbus and (F) the inside margin of the graft was fixed with 10-0 nylon anchoring sutures. (G) Tissue glue was used to firmly attach the mucosal graft to the bed. (H) After trimming excess tissue in the outside margin of the graft, it was sutured to the conjunctiva with continuous 10-0 nylon sutures to hold the graft onto the limbus. (I) Transient amniotic membrane transplantation was performed to protect the transplanted graft during the healing process.
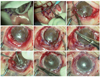
Fig. 2
Surgical outcomes of autologous oral mucosal graft transplantation in patients with limbal stem cell deficiency. (A) Prior to oral mucosal graft transplantation, cases 1 and 2 showed total limbal stem cell deficiency with a severe degree of inflammation and neovascularization. The other cases showed partial limbal stem cell deficiency (relevant areas are marked by dotted lines), and the clinical signs were less severe than those of total limbal stem cell deficiency. In cases 2, 3, and 5, corneal conjunctivalization, surface irregularity, and corneal opacity persisted despite amniotic membrane transplantation. (B) After 4 months, corneal neovascularization had fully regressed in cases 4 and 5 and decreased in cases 1 and 2. Corneal opacity decreased in cases 2 and 5 and was maintained in cases 3 and 4. In case 1, in which the patient received a permanent amniotic membrane transplantation, the eye presented increased opacity. In case 3, neovascularization was increased, and a persistent epithelial defect was apparent (marked by the dotted circle).
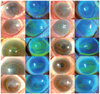
Fig. 3
Best-corrected visual acuity (BCVA) measured at 4 months after surgery. Visual acuity was improved in all patients, and the mean improvement of visual acuity was 0.526 ± 0.470 logarithm of the minimum angle of resolution (0.15 to 1.10 logarithm of the minimum angle of resolution). OS = oculus sinister; OD = oculus dexter; HM = hand motion; CF = count fingers.

Fig. 4
Final outcomes of oral mucosal graft transplantations in patients who received subsequent surgeries. (A) shows the status just prior to the subsequent surgery, and (B) shows the final corneal states. In case 1, the graft was conjunctivalized well 11 months following the oral mucosal graft transplantation, and a penetrating keratoplasty with phacoemulsification and intraocular lens implantation was performed. During the last follow-up 5 months after the subsequent surgery, corneal clarity was well maintained, and visual acuity had improved to 20 / 100. In case 3, the epithelial defect persisted, and the cornea had started to melt (marked by dotted circle). Transient and permanent amniotic membrane transplantations were performed to prevent the progression. The melted area was filled with amniotic membrane (marked by the double-lined dotted circle) and only a small epithelial defect remained (marked by the dotted circle).
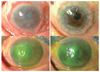
Table 1
Patient characteristics

Table 2
Clinical data of autologous oral mucosal graft for limbal stem cell deficiency










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download