This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
There is no standard method for confirming the immunogenicity of acellular pertussis vaccines. We tried to develop a local standard method for evaluating the immunogenicity of the three-component of acellular pertussis vaccines which was developed by a Korean local company.
Materials and Methods
The developed pertussis antigens (pertussis toxin, filamentous hemagglutinin, pertactin) were evaluated by in-house enzyme-linked immunosorbent assay (ELISA) using 189 negative sera, 25 positive sera, and 73 paired sera (pre- and post-Tdap [tetanus, diphtheria, and acellular pertussis] vaccinated sera). ELISA units were calculated by the reference line method, compared with World Health Organization reference sera, and the cut-off value was calculated using negative sera.
Results
When compared to National Institute for Biological Standards and Control control antigen (NIBSC) control antigens, the developed pertussis toxin (PT) and filamentous haemagglutinin (FHA) antigens were 203.48 and 61.60 IU/µg, respectively. Each in-house ELISA was established by validating the coefficients of variation % (PT, 11.53%; FHA, 8.60%; pertactin [PRN], 9.86%) obtained from the results of inter- and intra-assay variation. Also, the cut-off values of PT, FHA, and PRN were 11.65, 38.95, and 5.66 EU/mL, respectively. The distributions of antibody levels in paired showed that 93.15% (68/73) in anti-PT IgG, 97.26% (72/73) in anti-FHA IgG, and 100% in anti-PRN IgG were higher than a 100% increase after vaccination. Additionally, the values of 89.04% (65/73) in anti-PT IgG, 97.26% (72/73) in anti-FHA IgG, and 100% in anti-PRN IgG were below each cut-off point.
Conclusion
We established an in-house ELISA method using self-developed antigens, and these immunoassays have provided a way to standardize measuring the immunogenicity of newly developed vaccines, through single- and dual-serology.
Go to :

Keywords: Pertussis vaccine, Pertussis toxin, FHA protein, Pertactin, Enzyme-linked immunosorbent assay, Serology
Introduction
Bordetella pertussis is the main pathogen causing whooping cough (pertussis) that occurs after transmission of the bacteria in the form of airborne droplets between individuals. The incidence of this infection is highest in children under 5 years old, and the disease causes severe illness and death among neonates and infants. Pertussis vaccines have been very effective for over 40 years. However, re-emergence of pertussis was recently reported even in countries with high vaccination coverage [
123]. This increased incidence of adolescents and adults and familial transmission have been reported in many countries [
45]. Recently several risk causal factors have been suggested to contribute to the re-emergence of pertussis. First, protection after natural pertussis infection exposure is not life-long (typically 7 years) of the decreased immunity of pertussis vaccines may explain the shift in the incidence peak from school-age to adolescents/adults [
26]. Additionally, the genetic changes in circulating strains of
B. pertussis may have caused the re-emergence of pertussis [
278]. Thus, active immunization using pertussis vaccines is strongly recommended and a novel pertussis vaccine for overcoming these problems should be developed.
To prevent pertussis, diphtheria, and tetanus, diphtheria toxoid and acellular pertussis (DTaP) vaccines are administered to children under 7 years of age. DTaP vaccines consist of two, three, or five components of pertussis antigens (pertussis toxin, filamentous hemagglutinin, agglutinogens 1, 2) to reduce adverse events. Additionally, tetanus, diphtheria, and acellular pertussis (Tdap) vaccines which contain reduced doses of diphtheria and pertussis have been used as booster vaccines in adolescents and adults. In Korea, these acellular pertussis (aP) vaccines have been used since the 1990s, and all DTaP and Tdap vaccines are imported from foreign countries. However, pertussis vaccines are costly and the supply is unstable. Three-component acellular vaccines (DTaP and Tdap) have been developed by a domestic company to solve these problems. Phase I and IIa clinical studies using the newly developed Tdap vaccine were conducted in 2017.
Currently, there is no standard method for confirming the immunogenicity of aP vaccines. Thus, a standard method should be developed by each manufacturer or country while developing novel aP vaccines. Here, we developed a local standard method for evaluating the immunogenicity of the three-component (pertussis toxin, filamentous hemagglutinin, and pertactin [PRN]) in Tdap vaccine (GC Tdap) which was developed by GC Pharma (Yongin, Korea).
Go to :

Materials and Methods
Antigens and sera
Reference serum (World Health Organization [WHO] International Standard Pertussis Antiserum, National Institute for Biological Standards and Control control antigen [NIBSC] 06/140), reference pertussis toxin (PT; NIBSC JNIH-5) [
9], and filamentous haemagglutinin (FHA; NIBSC JNIH-4) antigens were purchased from the NIBSC (Hertfordshire, UK). PRN antigen was supplied by GC Pharma. The PT, FHA, and PRN antigens of the GC Tdap vaccine were compared to reference antigens by immunological analysis with reference serum. For immunological evaluation of these antigens, 189 negative sera obtained from healthy volunteers, 25 sera from patients with pertussis (confirmed by culture and polymerase chain reaction), and 73 paired sera from pre- and post-Tdap vaccinated sera (46 paired sera obtained from GC Tdap-vaccinated cases, group A; 27 paired sera obtained from GSK Tdap-vaccinated cases, group B) were used.
Immunological analysis
To evaluate and compare the pertussis antigens of the GC Tdap vaccine with the reference serum and antigens, an in-house sample diluted by 7-fold was examined by enzyme linked immunosorbent assay (ELISA) using a modified protocol [
10] as followings. Micro-titer plates (Nunc immuno plate, Thermo Fisher Scientific, Waltham, MA, USA) were coated with 100 µL PT (0.1 µg/mL), FHA (1.0 µg/mL), or PRN (1.0 µg/mL) in carbonate buffer (0.05 M Na
2HCO
3, pH 9.6). The plates were sealed and incubated at ±28℃ overnight (16–24 hours). All sera were diluted in incubation buffer (phosphate buffered saline pH 7.2 with 0.5% Tween 20 and 0.5% bovine serum albumin). Reference sera and control sera were diluted by two-fold (PT, l/240–1/30,720; FHA, 1/60–1/7,680; PRN, 1/120–15,360). After coating, the plates washed 3 times with wash buffer (0.145 M NaCl, 0.5% Tween 20), and then incubated with incubation buffer for 1 hour. Reference, control, and sample sera (50 µL) were incubated for 2 hours at ±28℃. Goat serum anti-human IgG conjugated to alkaline phosphatase (AP; Kirkegaard and Perry, Gaithersburg, MD, USA) diluted in incubation buffer was added and incubated at 28℃ overnight. After washing, the AP substrate PNPP (Sigma-Aldrich, St. Louis, MO, USA) in 1 M Tris-HCl pH 9.8 with 0.3 mM MgCl
2 was added, after which the reaction was stopped by adding 5 N NaOH. Absorbance was measured at 405 nm with a SpectraMax 190 (Molecular Devices, Sunnyvale, CA, USA). All results were validated according to the following criteria: reagent control (blank) must be <0.200, antibody control must be <0.150, and standard curve must have a fit of r
2≥0.98.
The variance in the ELISA results was used to calculate the various coefficients of variation (CV), defined as the respective standard deviation (SD) divided by the overall mean, and then the CV was used to determine the reproducibility of ELISA. When the inter-assay CV against each pertussis antigen was below 15%, the ELISA was accepted as an evaluation method.
Calculation of ELISA unit and determination of cut-off value
A dose-response curve was calculated from the natural logarithm of the absorbance value (
A405) against the log
2 serum dilution. Additionally, ELISA units were calculated using the reference line method as previously described [
11]. The lower limit of detection was defined as the lowest amount of antibody detected and was determined to be 1 ELISA unit (EU). To calculate the cut-off value of each antigen, sera from normal volunteers (n=189) were used. The cut-off value was calculated as the mean±2SD as previously described [
12]. By comparison with control antigens (NIBSC PT and FHA antigen), antigens in the GC Tdap vaccine were assessed using each calculated cut-off value.
Comparison by single-sample serology and dual-sample serology
Clinical sera (n=25) were tested by in-house ELISA and with a
Bordetella pertussis IgG ELISA kit (IBL International GmbH, Hamburg, Germany). Based on the results (positive, equivocal, and negative) using the cut-off values, in-house ELISA results were compared to those of the commercial kit. Based on a ≥100% increase in antibody concentration, dual sample serology was tested to determine the sensitivity and specificity of antigens [
1314]. In each in-house ELISA, 73 paired sera from pre- and post-vaccinated sera were used, and then each result was compared to those of NIBSC antigens.
Statistical analysis
Data were compared by the unpaired t test. All hypotheses were two-tailed and considered significant at the p<0.01 level.
Ethics statement
The study protocol was approved by the Institutional Review Board of the Catholic Medical Center (IRB No. KC12TNS10283 and XC09TIMI008K). Informed consent was confirmed by the IRB.
Go to :

Results
Comparing pertussis antigens by reference serum, establishing in-house ELISA, and determining cut-off values
The concentration of antigens in the GC Tdap was determined from a standard curve using reference international standard pertussis antiserum (06/140). The final concentrations were 0.1 µg/mL of PT, 1 µg/mL of FHA, and 0.5 µg/mL of PRN.
Using the reference line method with WHO international standard pertussis antiserum (06/140) and NIBSC control antigens, the PT and FHA antigens of the GC Tdap vaccine were 203.48 and 61.60 IU/µg, respectively, compared to those of NIBSC antigens. However, PRN antigens could not be compared because of no controls were available. Each in-house ELISA was established by validating the CV% (PT CV%, 11.53%; FHA, 8.60%; PRN, 9.86%) obtained from the results of 15 consecutive tests including analysis of inter- and intra-assay variation.
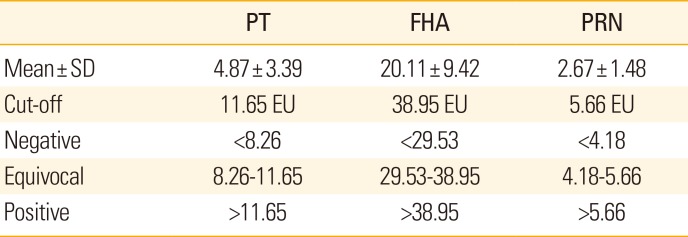
Each cut-off value was determined from the results of in-house ELISA for the sera of the negative groups (n=189). The cut-off values of PT, FHA, and PRN were 11.65, 38.95, and 5.66 EU/mL, respectively (
Table 1). In this population, the mean concentration of anti-FHA IgG in healthy volunteers was higher than in those of anti-PT and PRN IgG. The interpretation criteria (negative, equivocal, and positive) were determined from the cut-off value and SD (
Table 1). There was no significant difference in results compared to the in-house ELISA using NIBSC antigens (only PT and FHA).
Table 1
Cut-off values and interpretation of each in-house ELISA results obtained from the sera of volunteer groups (n=189)
|
PT |
FHA |
PRN |
|
Mean±SD |
4.87±3.39 |
20.11±9.42 |
2.67±1.48 |
|
Cut-off |
11.65 EU |
38.95 EU |
5.66 EU |
|
Negative |
<8.26 |
<29.53 |
<4.18 |
|
Equivocal |
8.26-11.65 |
29.53-38.95 |
4.18-5.66 |
|
Positive |
>11.65 |
>38.95 |
>5.66 |

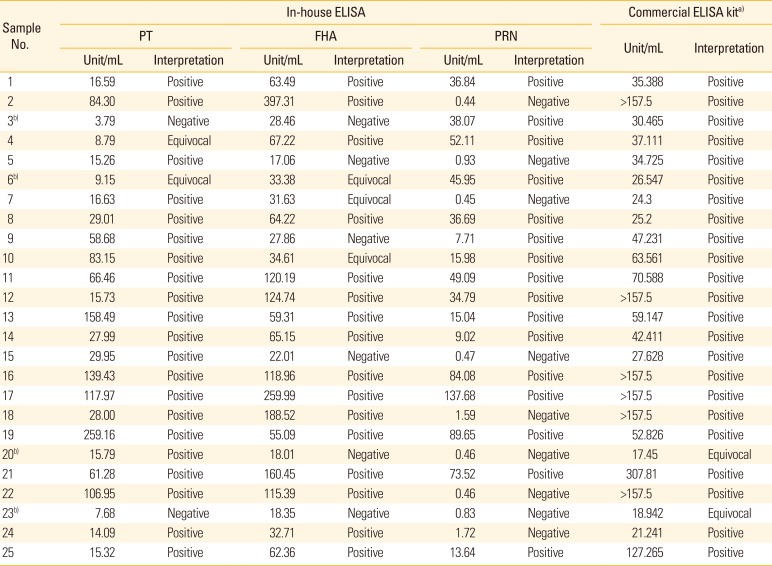
Verification of established in-house ELISA
The in-house ELISA established in this study was verified by comparison of the results obtained using commercial ELISA kit for 25 clinical sera obtained from patients with pertussis to detect IgG against
B. pertussis PT, FHA, and lipopolysaccharide (LPS). Among the samples (n=23) showing positive results for the commercial kit, 21 samples showed anti-PT or/and anti-FHA positivity in the in-house ELISA results (
Table 2). However, the results of equivocal samples (n=2) did not coincide (
Table 2); this may be because of the activity of other antigens (anti-LPS). Anti-PRN IgG detection by the in-house ELISA showed a positivity of 68.0% (17/25) in patients with pertussis, although comparison with the commercial kit or a control antigen was not possible. These results suggest that the cut-off values determined in this study can be used as criteria for determining antibody production at the single serology level in vaccine studies.
Table 2
Comparison of in-house ELISA and commercial ELISA kit using 25 clinical sera obtained from patients with pertussis
|
Sample No. |
In-house ELISA |
Commercial ELISA kita)
|
|
PT |
FHA |
PRN |
Unit/mL |
Interpretation |
|
Unit/mL |
Interpretation |
Unit/mL |
Interpretation |
Unit/mL |
Interpretation |
|
1 |
16.59 |
Positive |
63.49 |
Positive |
36.84 |
Positive |
35.388 |
Positive |
|
2 |
84.30 |
Positive |
397.31 |
Positive |
0.44 |
Negative |
>157.5 |
Positive |
|
3b)
|
3.79 |
Negative |
28.46 |
Negative |
38.07 |
Positive |
30.465 |
Positive |
|
4 |
8.79 |
Equivocal |
67.22 |
Positive |
52.11 |
Positive |
37.111 |
Positive |
|
5 |
15.26 |
Positive |
17.06 |
Negative |
0.93 |
Negative |
34.725 |
Positive |
|
6b)
|
9.15 |
Equivocal |
33.38 |
Equivocal |
45.95 |
Positive |
26.547 |
Positive |
|
7 |
16.63 |
Positive |
31.63 |
Equivocal |
0.45 |
Negative |
24.3 |
Positive |
|
8 |
29.01 |
Positive |
64.22 |
Positive |
36.69 |
Positive |
25.2 |
Positive |
|
9 |
58.68 |
Positive |
27.86 |
Negative |
7.71 |
Positive |
47.231 |
Positive |
|
10 |
83.15 |
Positive |
34.61 |
Equivocal |
15.98 |
Positive |
63.561 |
Positive |
|
11 |
66.46 |
Positive |
120.19 |
Positive |
49.09 |
Positive |
70.588 |
Positive |
|
12 |
15.73 |
Positive |
124.74 |
Positive |
34.79 |
Positive |
>157.5 |
Positive |
|
13 |
158.49 |
Positive |
59.31 |
Positive |
15.04 |
Positive |
59.147 |
Positive |
|
14 |
27.99 |
Positive |
65.15 |
Positive |
9.02 |
Positive |
42.411 |
Positive |
|
15 |
29.95 |
Positive |
22.01 |
Negative |
0.47 |
Negative |
27.628 |
Positive |
|
16 |
139.43 |
Positive |
118.96 |
Positive |
84.08 |
Positive |
>157.5 |
Positive |
|
17 |
117.97 |
Positive |
259.99 |
Positive |
137.68 |
Positive |
>157.5 |
Positive |
|
18 |
28.00 |
Positive |
188.52 |
Positive |
1.59 |
Negative |
>157.5 |
Positive |
|
19 |
259.16 |
Positive |
55.09 |
Positive |
89.65 |
Positive |
52.826 |
Positive |
|
20b)
|
15.79 |
Positive |
18.01 |
Negative |
0.46 |
Negative |
17.45 |
Equivocal |
|
21 |
61.28 |
Positive |
160.45 |
Positive |
73.52 |
Positive |
307.81 |
Positive |
|
22 |
106.95 |
Positive |
115.39 |
Positive |
0.46 |
Negative |
>157.5 |
Positive |
|
23b)
|
7.68 |
Negative |
18.35 |
Negative |
0.83 |
Negative |
18.942 |
Equivocal |
|
24 |
14.09 |
Positive |
32.71 |
Positive |
1.72 |
Negative |
21.241 |
Positive |
|
25 |
15.32 |
Positive |
62.36 |
Positive |
13.64 |
Positive |
127.265 |
Positive |

Evaluation of in-house ELISA in vaccine study
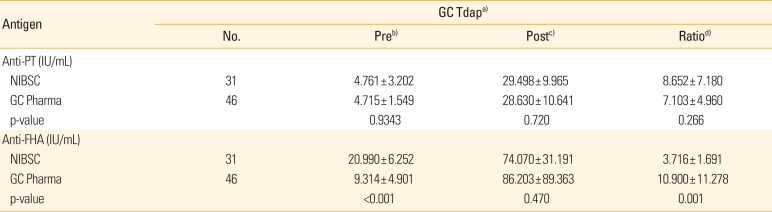
Each in-house ELISA was validated using vaccinated sera and then compared according to the type of antigen (PT & FHA from NIBSC & GC Tdap vaccine). In anti-PT IgG analysis, there were no significant differences between the results for the type of antigens (NIBSC & GC Tdap vaccine) (
Table 3). However, the anti-FHA IgG results showed significant differences in the pre-vaccinated sera (p<0.001) and an increased ratio (p=0.001) after vaccination, but not in the post-vaccinated sera (p=0.470), between each method (
Table 3).
Table 3
Comparison of results of each in-house ELISA in paired sera (pre- and post-vaccinated sera) using NIBSC and GC Tdap vaccine antigens
|
Antigen |
GC Tdapa)
|
|
No. |
Preb)
|
Postc)
|
Ratiod)
|
|
Anti-PT (IU/mL) |
|
|
|
|
|
NIBSC |
31 |
4.761 ± 3.202 |
29.498 ± 9.965 |
8.652 ± 7.180 |
|
GC Pharma |
46 |
4.715 ± 1.549 |
28.630 ± 10.641 |
7.103 ± 4.960 |
|
p-value |
|
0.9343 |
0.720 |
0.266 |
|
Anti-FHA (IU/mL) |
|
|
|
|
|
NIBSC |
31 |
20.990 ± 6.252 |
74.070 ± 31.191 |
3.716 ± 1.691 |
|
GC Pharma |
46 |
9.314 ± 4.901 |
86.203 ± 89.363 |
10.900 ± 11.278 |
|
p-value |
|
<0.001 |
0.470 |
0.001 |

In a dual-serology study (pre- and post-vaccinated sera by Tdap of A & B company), there were a significant increase (p<0.001) in individual immunogenicity after vaccination (
Fig. 1). The distributions of antibody levels in paired sera showed that 6.85% (5/73) in anti-PT IgG, 1.37% (1/73) in anti-FHA IgG, and 0% in anti-PRN IgG were lower than a 100% increase after vaccination. Additionally, the values of 10.96% (8/73) in anti-PT IgG, 1.37% (1/73) in anti-FHA IgG, and 0% in anti-PRN IgG were below each cut-off point (
Fig. 2). These results suggest that the in-house method is valuable for vaccine studies using clinical specimens with dual-serology or single-serology.
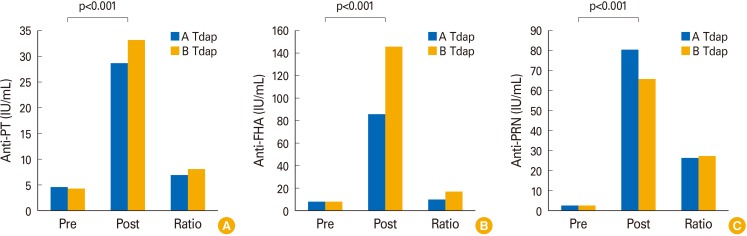 | Fig. 1Analysis of anti-pertussis antigens (A, PT; B, FHA; C, PRN), IgG in the pre- and post-vaccinated sera group, and increased ratio between A and B Tdap vaccination group. PT, perussis toxin; FHA, filamentous haemagglutinin; PRN, pertactin; Tdap, tetanus, diphtheria, and acellular pertussis.
|
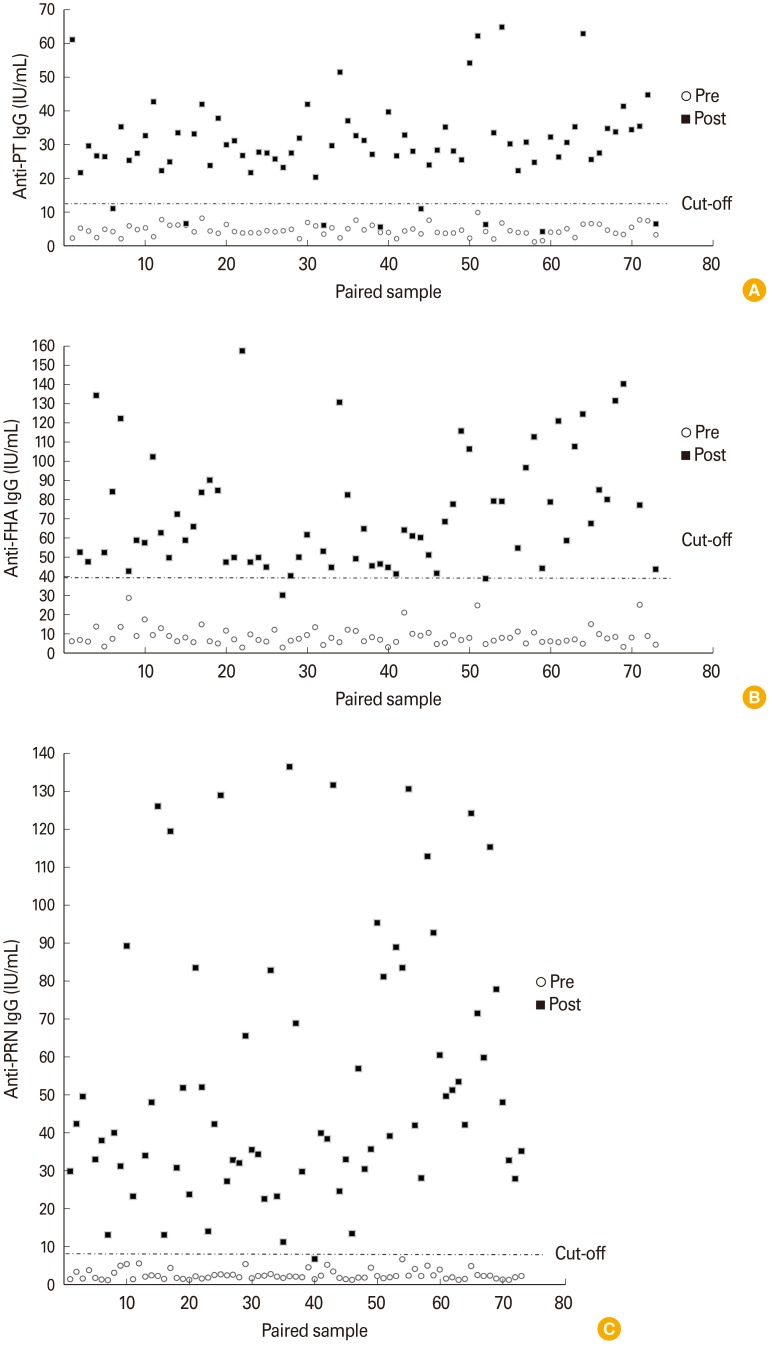 | Fig. 2Results of dual-serology (paired pre- and post-vaccinated sera) by established in-house enzyme-linked immunosorbent assay. X-axis indicates paired sample, while y-axis indicates anti-PT IgG (A), anti-FHA IgG (A), and anti-PRN IgG (C), respectively. Black squares indicate post-vaccinated sera, while blank circles indicate pre-vaccinated sera. PT, perussis toxin; FHA, filamentous haemagglutinin; PRN, pertactin
|
Go to :

Discussion
aP vaccines have been used since the late 1990s worldwide because they are comparatively safer than whole cell pertussis vaccines. The three components in aP vaccines are an attenuated toxin component such as PT, a protein associated with bacterial adhesion such as FHA or PRN. PT is a secretory toxin that can be divided into five subunits, largely the A and B parts [
1516]. Part B attaches to the host cell and serves to transport part A into the host cell. Part A can destroy the function of the host cell through its ADP-ribosylating activity [
1718]. This toxin is not secreted by
B. parapertussis strains. FHA, a major adhesin molecule, contributes to pathogenicity by attaching to sugar components, heparin, integrin CR3 site, macrophages, and epithelial cells [
192021], and PRN plays an important role in macrophage adhesion [
2223]. These factors are involved in host antibody formation for protection. Therefore, a standardized ELISA assay method is critical for vaccine evaluation, as it can measure the effect of a vaccine by analyzing the antibodies of PT, FHA, and PRN.
No control antigen or standard antibody is available in South Korea. Additionally, a standard PRN antigen has not established worldwide. Thus, we developed an in-house ELISA method using NIBSC standard control antigens (NIBSC PT & FHA), NIBSC standard control serum, and the PRN antigen of a domestic developed acellular vaccine in this study.
In these assays, the CVs of PT, FHA, and PRN were 11.53%, 8.60%, and 9.86%, respectively. Because all measured CVs were below 15%, this method is reproducible. This established method was used to determine the negative and positive criteria of each pertussis antigen by measuring the cut-off level using normal human samples. These cut-off levels against each antigen were applied to the sera of 25 patients with pertussis. Although conventional commercial kits are limited to differentiate the positivity of each test pertussis antigen, the results were significantly matched except for in 4 cases when these results were compared by using conventional pertussis diagnostic test kit approaches (
Table 2). The cut-off point is a reference criterion for the immunological diagnosis of pertussis in countries with a high vaccination coverage [
14], but it is also an important factor in determining the effect of the Tdap vaccine in adults. While there were no significant differences in anti-PT IgG analysis, the anti-FHA IgG results between the type of antigens (NIBSC & GC Tdap vaccine) showed significant differences in the pre-vaccinated sera and an increased ratio (
Table 3). This may be because of differences in the detection ability at low concentrations of anti-FHA IgG. However, because there was no significant difference in the post-vaccinated group (anti-FHA IgG, p=0.470) (
Table 3), it is possible to verify the vaccine effect using the same approach as used in single serology.
In a dual-serology study (pre- and post-vaccinated sera by Tdap of A & B company), the antibody titers of pertussis antigens before vaccination were all below the cut-off value, and there were a significant increase (p<0.001) in individual immunogenicity after vaccination (
Figs. 1,
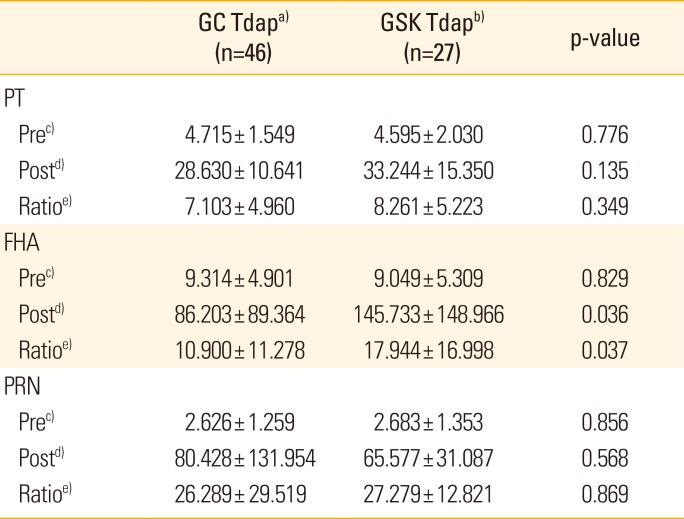
2). There was no difference in the anti-PT and anti-PRN IgG formation in the Tdap vaccine group of the two companies (p=0.135 and p=0.568, respectively), and there was a difference in anti-FHA IgG immunogenicity, but the difference was not significant (p>0.01) (
Table 4). In the distribution of anti-pertussis antigen IgG in pre- and post-vaccinated sera, the cut-off point can be a useful tool for evaluating immunogenicity after vaccination with a single serology test. These results suggest that the established in-house ELISA method and cut-off points in this study are valuable for confirming individual immunogenicity of each pertussis antigen after vaccination.
Table 4
Comparison of results of in paired sera (pre- and post-vaccinated
sera) from groups A and B
|
GC Tdapa) (n=46) |
GSK Tdapb) (n=27) |
p-value |
|
PT |
|
|
|
|
Prec)
|
4.715±1.549 |
4.595±2.030 |
0.776 |
|
Postd)
|
28.630±10.641 |
33.244±15.350 |
0.135 |
|
Ratioe)
|
7.103±4.960 |
8.261±5.223 |
0.349 |
|
FHA |
|
|
|
|
Prec)
|
9.314±4.901 |
9.049±5.309 |
0.829 |
|
Postd)
|
86.203±89.364 |
145.733±148.966 |
0.036 |
|
Ratioe)
|
10.900±11.278 |
17.944±16.998 |
0.037 |
|
PRN |
|
|
|
|
Prec)
|
2.626±1.259 |
2.683±1.353 |
0.856 |
|
Postd)
|
80.428±131.954 |
65.577±31.087 |
0.568 |
|
Ratioe)
|
26.289±29.519 |
27.279±12.821 |
0.869 |

In conclusion, we established an in-house ELISA method using self-developed vaccine raw materials by comparing immunogenicity test methods with the WHO standard antibody and control antigen. The developed test and cut-off point were verified by using pertussis-positive sera and vaccine sera. There are some limitations to using the standard antigen and standard serum. Particularly, because a standard antigen for PRN is not available, studies are needed to establish the standard antigens and standard antibodies in South Korea. Verification of pertussis antigens of B. pertussis strains with polymorphisms and undergoing epidemiological changes should be conducted after some time. When the immunodynamic changes in pertussis are large, a new standard antibody and control antigen should be developed to re-establish the reference unit, cut-off limit, and so on. Our method can be used to establish a domestic standard pertussis immunoassay using the sera of patients with pertussis and vaccine sera and suggests the necessity of establishing domestic standard sera and an antigen.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download