INTRODUCTION
MATERIALS AND METHODS
Adzuki extract
Preparation of Aβ solution
Thioflavin T fluorescence assay
Transmission electron microscopy
Fly stocks and culture
ELISA analysis
Climbing assay
Single-cycle training and memory assays
Longevity assay
Statistical analysis
RESULTS
Effect of adzuki extract on in vitro Aβ42 aggregation
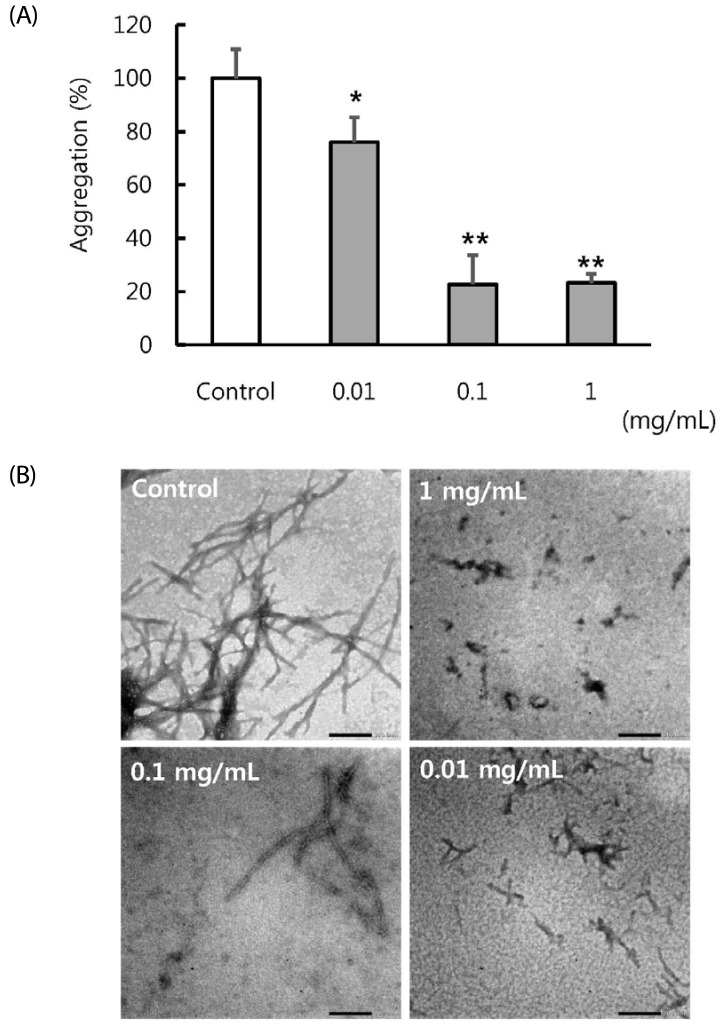 | Fig. 1Effect of adzuki bean extract on in vitro Aβ42 aggregation.(A) The Aβ42 solution was incubated at 37℃ for 24 h in the absence (white bar) or presence (black bar) of various concentrations of the adzuki extract (0.01–1 mg/mL). The samples were evaluated by examining ThT fluorescence. Emission wavelength was monitored at 490 nm and excitation at 446 nm. The results are expressed as Means±SEM for three independent determinations. *
P < 0.05, **
P < 0.01 compared with the control, n = 3. (B) TEM results; the Aβ42 solution was incubated at 37℃ for 24 h in the absence or presence of indicated concentrations of the adzuki extract. In each case, the scale bar represents 200 nm.
|
Intake of adzuki extract reduces the Aβ42 level in the brain of Aβ42-overexpressing flies
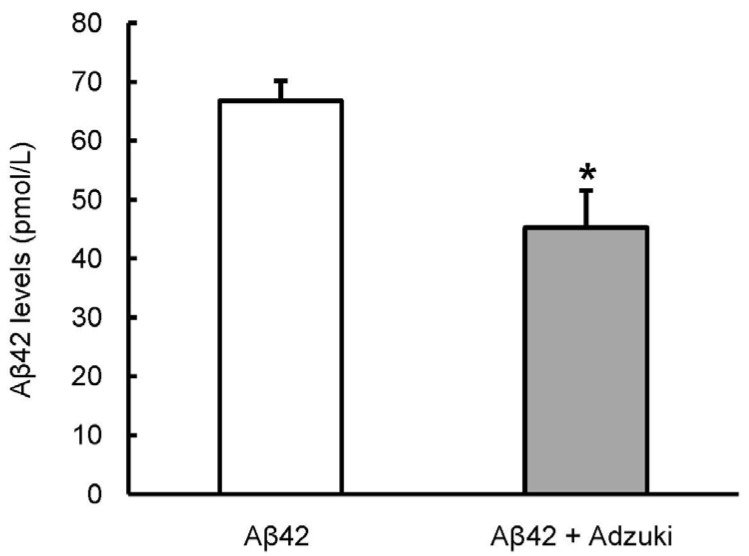 | Fig. 2Intake of adzuki bean extract reduces the Aβ42 levels in the brain of Aβ42-overexpressing flies.Aβ42-overexpressing flies were grown on regular medium (Aβ42) or medium containing adzuki extract (Aβ42+Adzuki). At 15 days old, Aβ42 levels in fly head lysates were quantified by ELISA. Results are expressed as Means±SEM for three independent determinations. *
P < 0.05 compared with Aβ42 (grown on regular medium) n = 60.
|
Intake of adzuki extract increases the locomotor activity of Aβ42-overexpressing flies
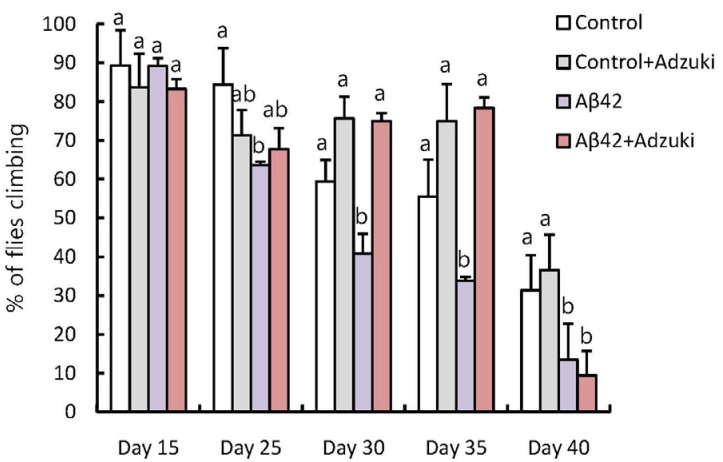 | Fig. 3Intake of adzuki bean extract increases the locomotor activity of Aβ42-overexpressing flies.Control flies (carrying the Aβ42 transgene but not expressing it) were grown on regular medium (control) or on medium containing adzuki extract (Control+Adzuki), and Aβ42-overexpressing flies were grown on regular medium (Aβ42) or medium containing adzuki extract (Aβ42+Adzuki). All fly groups underwent climbing assays in the indicated days after eclosion. Results show the percentage of flies climbing to the top of the vial after 18 seconds. The results are expressed as Means±SEM for three independent determinations. Means with different letters are significantly different, P < 0.05, whereas means with similar letters are not different from each other; n > 90.
|
Intake of adzuki extract increases the learning and memory abilities of Aβ42-overexpressing flies
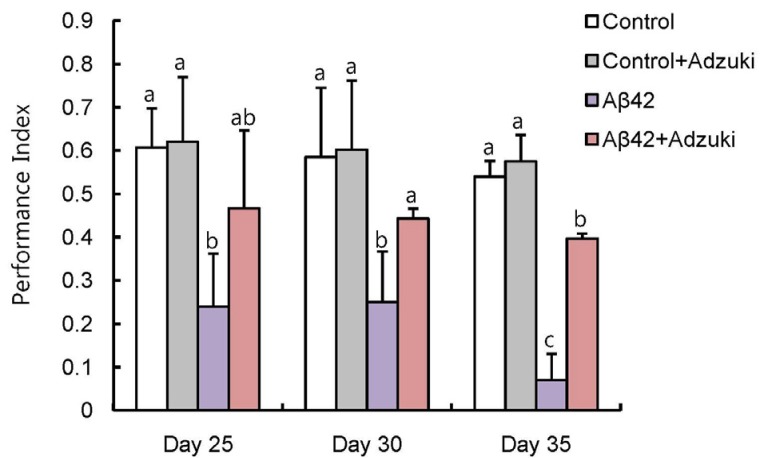 | Fig. 4Intake of adzuki bean extract increases the learning and memory abilities of Aβ42-overexpressing flies.Control flies (carrying the Aβ42 transgene but not expressing it) grown on regular medium (Control) or medium containing adzuki extract (Control+Adzuki) and Aβ42-overexpressing flies grown on regular medium (Aβ42) or medium containing adzuki extract (Aβ42+Adzuki) were tested using single-cycle olfactory training and memory assays in the indicated days after eclosion. The results are expressed as Means±SEM for three independent determinations. Means with different letters are significantly different, P < 0.05, whereas means with similar letters are not significantly different, n > 300.
|
Intake of adzuki extract increases the lifespan of Aβ42-overexpressing flies
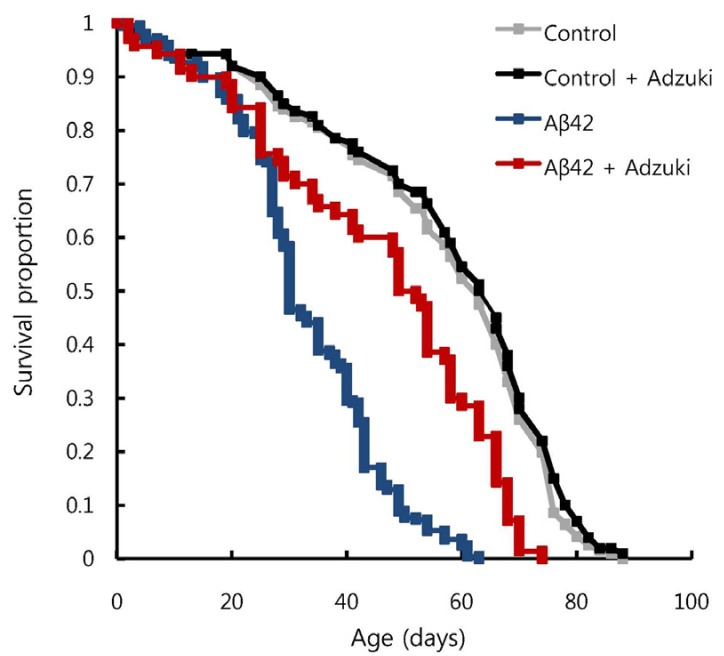 | Fig. 5Intake of adzuki bean extract increases the lifespan of Aβ42-overexpressing flies.Survival rates of control flies (carrying the Aβ42 transgene but not expressing it) grown on regular medium (Control) or medium containing adzuki extract (Control+Adzuki) and Aβ42 flies grown on regular medium (Aβ42) or medium containing adzuki extract (Aβ42+Adzuki) were analyzed using survival assays. Kaplan-Meier and log-rank analyses revealed that median survival times for Aβ42-overexpressing flies grown on regular medium and grown on medium containing adzuki extract were significantly different (32.8 days and 45.1 days, respectively, P < 0.001, n > 200).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download