INTRODUCTION
Primary cardiac tumors are rare. Roughly 75% of cardiac tumors are benign; half of these are myxomas, which have an approximate incidence of 0.5-1.0 cases per million inhabitants per year. Myxomas occur in all age groups and in both sexes, although they occur more often in women between the third and sixth decades of life.
1)2)
Although histologically benign, in some cases myxomas are lethal due to impaired cardiac dynamics and thromboembolic potential.
3) When tumors are diagnosed and resected in a timely manner, they lose their deleterious potential and patients are cured, except for rare cases of recurrence.
4)5) Patients have survival similar to that of a normal person, and there is no impact on quality of life.
With the availability of echocardiography among initial ancillary tests performed in cardiovascular practice, tumor detection has increased. This has resulted in increased publication of case reports and patient series on cardiac tumors.
There are few local reports describing the surgical outcome of these tumors.6)7) In this study, we present the 20-year experience of our institution in diagnosis and long-term follow-up of patients with cardiac myxomas.
The goals of this study were to assess the clinical presentation of cardiac myxomas and their correlation with echocardiographic features and describe the perioperative results and long-term outcome of surgically treated patients.
METHODS
Patients
An observational, prospective study of 53 patients with cardiac myxomas operated at Hospital Argerich was conducted. Patients were followed clinically with echocardiography from 1993 until 2013.
Our Institutional Review Board approved this study and patients agreed to this prospective study. All patients signed Informed Consent.
All patients underwent transthoracic echocardiographic studies performed with the patient in the left lateral position using a Hewlett-Packard Sonos 5500 system (Philips Medical Systems, Bothell, WA, USA) in the first series and later using a Vivid 7 machine (GE Medical System, Horten, Norway) according to recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.
8)
In patients with a suboptimal transthoracic window that did not allow assessment of tumor characteristics, a transesophageal echocardiography (TEE) was performed following the recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists.
9)
Myxomas were classified according to their surfaces
10) as smooth (with regular borders) (
Figure 1A, B) or villous (with irregular borders, multilobulated, and/or gelatinous) (
Figure 1C, D).
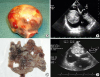 | Figure 1Smooth myxoma: surgical specimen (A), transesophageal echocardiogram (B). Villous myxoma: surgical specimen (C), transesophageal echocardiogram (D).
|
Myxomas were classified according to their intratumor appearance as homogeneous or heterogeneous, which was defined by the presence or absence of hypo- or hyper-echogenic areas identified by echocardiography.
Myxomas were defined as obstructive if the mitral or tricuspid area was smaller than 2 cm2 calculated by pressure half-time, and as small or large according to whether the diameter was above or below 4 cm.
In patients who underwent surgery and it was necessary to rule out the presence of associated coronary disease, a coronary angiography was performed prior to the surgical intervention.
At follow up serial echocardiographic studies were performed to search for the presence of tumor recurrence.
Demographic data, clinical background, preoperative history, surgical data, pathology data, and immediate post-operative outcome were evaluated. Clinical and echocardiographic assessment was ascertained for long-term follow-up. In some cases, telephone follow-up was also performed.
Statistical analysis
Epi info 3.4.3 was used for all statistical calculations. Discrete variables were compared with a chi-squared or Fisher's Exact test, as appropriate. Normally distributed continuous variables were assessed with a Student's t-test and non-Gaussian distributions were assessed with a Mann-Whitney test. A p < 0.05 was considered statistically significant, and Kaplan-Meier curves were used for survival analysis.
RESULTS
Baseline characteristics
Mean age was 53 ± 16.8 years (range: 17–90 years); 33 patients (62.3%) were women. The age distribution was homogeneous with a predominance of women, except in the range between 51 and 60 years, where male sex prevailed. The most frequent comorbidity was hypertension (34%) followed by diabetes (9.3%), coronary artery disease (9.3%), and infectious disorders (9.3%) (
Table 1).
Table 1
Baseline Characteristics and Tumor Location

|
Age (years) |
53 ± 16.8 |
|
Sex |
|
|
Male |
20 (37.7) |
|
Female |
33 (62.3) |
|
Medical history |
|
|
Hypertension |
18 (34) |
|
Atrial fibrillation |
5 (9.3) |
|
Diabetes |
5 (9.3) |
|
Coronary artery disease |
5 (9.3) |
|
Infectious disorders |
5 (9.3) |
|
Cancer |
4 (7.5) |
|
Psychiatric disease |
3 (5.6) |
|
Inflammatory disorders |
2 (3.7) |
|
Hypothiroidism |
2 (3.7) |
|
Clinical manifestations |
|
|
Dyspnea |
30 (56.6) |
|
Constitutional symptoms |
14 (26.4) |
|
Embolic symptoms |
13 (24.5) |
|
|
Brain |
10 (77) |
|
|
Pulmonary |
3 (23) |
|
Incidental |
9 (17) |
|
Tumor location |
|
|
LA |
41 (77.4) |
|
|
Fossa ovalis |
26 (63.4) |
|
|
Atrial septum (no fossa ovalis) |
12 (29.3) |
|
|
Other atrial sector |
3 (7.3) |
|
RA |
9 (17) |
|
RV |
1 (1.9) |
|
LV |
1 (1.9) |
|
Bi-A |
1 (1.9) |

Clinical manifestations
When myxomas were not an incidental echocardiographic finding and patients reported symptoms, the median time since symptom onset until diagnosis was 14 weeks, Confidence Interval (CI): 12–48 weeks.
Most common symptoms were cardiovascular in origin, mainly dyspnea (56.6%), followed by constitutional symptoms (26.4%) (including fever, asthenia, anemia, and weight loss) and embolic symptoms (24.5%). Only 9 tumors (17%) were incidental echocardiographic findings in an echocardiogram ordered for other reasons (
Table 1).
Echocardiographic characteristics
Transthoracic echo was performed in all cases. In 34 patients (62%) in whom the ultrasound window did not allow adequate assessment of tumor characteristics, the exam was completed with a transesophageal echo.
Location
A total of 77.4% of cases were located in the left atrium, followed by the right atrium (17%), right ventricular outflow tract (1.9%), and mitral valve (1.9%). A rare patient had 2 myxomas, one in each atrium on both sides of the fossa ovalis (
Figure 2).
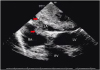 | Figure 2Biatrial villous myxoma (arrows). Transesophageal echocardiogram, 4-chamber view. LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
|
When tumors were located in the left atrium, most were attached to the fossa ovalis (63.4%) by a pedicle; the rest were in another area of the atrial septum (29.3%) or in another atrial sector (7.3%) (
Table 1).
Size and mobility
Mean tumor size was 4.76 ± 2.00 cm in the long axis (range 1.00–8.20 cm) and 3.50 ± 1.48 cm in the short axis (range 0.78–6.00 cm).
Tumors were generally mobile (88.7%, n = 47), and more than half went beyond the valve plane (58.5%, n = 31). When tumors occupied atrial cavities, there was mild dilation of the respective atrial chamber (mean area: 23.5 ± 4.4 cm2 for left-sided tumors and 23.6 ± 6.3 cm2 for right-sided tumors).
Thirty tumors (58.5%) presented with mitral or tricuspid valve obstruction, according to the valve involved, with an area estimated by a pressure halftime of 1.04 ± 0.27 cm2 and an average mean gradient of 8.71 ± 4.51 mmHg. Mild or moderate mitral regurgitation was found in 44% (19 of 43) of left-sided myxomas, whereas mild tricuspid regurgitation was found in 40% (4 of 10) of right-sided myxomas. In cases with obstructive tumors, pulmonary artery systolic pressures were higher compared to cases with non-obstructive tumors (50 ± 24 mmHg vs. 33 ± 18 mmHg, p < 0.01).
Cardiovascular symptoms were associated with large myxomas (77% vs. 23%, p < 0.05) and obstructive myxomas (65% vs. 35%, p < 0.01). All patients with constitutional symptoms had tumors larger than 4 cm (100% vs. 0%, p < 0.01) and most were obstructive (86% vs. 14%, p < 0.01). Patients in whom the tumor was an incidental echocardiographic finding had smaller myxomas (77.8% vs. 22.2%, p < 0.01).
Morphologic features
The echocardiographic appearance of tumors was classified as homogeneous in 47.2% (n = 25) of patients, while the remaining 52.8% (n = 28) were heterogeneous.
According to echocardiography appearance, 67.9% (n = 36) of tumors had a smooth surface (
Movie 1) and the remaining 17 patients (32.1%) had a villous surface (
Movie 2). Patients with embolic manifestations had a villous surface (100% vs. 0%, p < 0.01), but there were no differences in intratumor appearance or size.
Treatment
One patient with a large villous myxoma located in the right atrium died suddenly while waiting for surgery due to massive pulmonary thromboembolism. Five patients did not undergo surgery for various reasons: 3 patients due to advanced age (2 octogenarians and 1 nonagenarian), 1 because of high surgical risk, and 1 because the patient rejected the surgical option.
The remaining 47 patients (88.67%) had surgical resection, and median time from echocardiographic diagnosis to surgery was 12 days (CI: 6–31 days).
Coronary angiography was performed in 35 patients (74.5%) prior to surgery, and only 5 patients (10.6%) had significant coronary disease requiring coronary revascularization in addition to myxoma resection. Mitral or tricuspid valve repair was performed in 6 patients (12.7%).
Surgery consisted of tumor resection using the right atrial approach followed by the trans-septal approach (76.6%, n = 36) for tumors located in some portion of the atrial septum. Right-sided tumors were resected exclusively through the right atrial approach. During the immediate postoperative period, 21 patients (44.7%) had at least one complication (
Table 2). In the immediate postoperative period, arrhythmias were the most common complications (33.3%, n = 7), followed by infections (19%, n = 4), vasoplegic syndrome (19%, n = 4), and pericardial effusion (14.2%, n = 3). All patients had a good outcome and there were no hospital deaths.
Table 2
Postoperative complications

|
Patients with complications, % (n) |
21/47 (44.7) |
|
All complications |
21 |
|
Arrhythmias |
7 (33.3) |
|
Infections |
4 (19) |
|
Vasoplegic syndrome |
4 (19) |
|
Pericardial Effusion |
3 (14.2) |
|
Acute renal failure |
2 (9.5) |
|
Atrial septal defect |
2 (9.5) |
|
Stroke |
1 (4.8) |
|
Pulmonary Embolism |
1 (4.8) |

Pathology specimens were obtained in all surgical patients, and the diagnosis of myxoma was confirmed. Additionally, detailed histological information was obtained in 31 patients (61.9%) (
Figure 3).
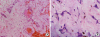 | Figure 3Histology of left atrial myxoma. Optic microscopy. (A) Hematoxylin–eosin stain. Proliferation of spindle and stellate mesenchymal cells around thin-walled vessels, with recent hemorrhage and macrophages filled with hemosiderin. Myxoid matrix with thin capillaries. (B) Microphotograph showing myxoid background, proliferation of star shaped cells, thin-walled vessels and macrophages with hemosiderin.
|
Calcification was present in 22.6% of cases, necrotic foci were present in 22.5% of cases, and tumor hemorrhage was present in 51.6% of cases. All pathology specimens with calcification had a heterogeneous appearance on echocardiography (100% vs. 0%, p < 0.001) (
Figure 4) that was unrelated to necrotic or hemorrhagic foci.
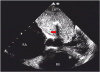 | Figure 4Left atrial myxoma with intra-tumor calcification. Transesophageal echocardiogram, 4-chamber view. The presence of calcification inside the tumor causes acoustic shadowing (arrow). LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
|
Follow-up
Long-term follow up was performed in a single center, which is a strength of this study. Echocardiographic follow-up was available in 98.1% of patients. Median follow-up was 8.01 years, interquartile range (IQR): 4.21–11.12 years), and survival of surgical patients was 87.9% at 10 years (IQR: 68.9–96%).
Only one patient (1.88%) had tumor recurrence 9 years after surgery, which required reintervention. He did not exhibit clinical characteristics of the familial form (Carney Complex Syndrome).
Limitation
The echocardiographic follow-up available in 98.1% of patients could be considered a minor limitation of this study.
DISCUSSION
The most important finding of our study was that 76.5% of villous myxomas presented with embolic episodes, whereas no embolic events occurred in patients with smooth surface myxomas. Smooth tumors are larger, occur with obstructive symptoms, and benefit from elective surgery, whereas villous myxomas entailed a high embolic risk and required urgent surgical treatment.
Primary cardiac tumors are a rare disorder; myxoma is the most frequent primary cardiac tumor. The sporadic type is seen most often and comprises about 93% of all varieties. Women are affected more often than men, and the average age of patients is approximately 50 years. Tumors are generally solitary, most often located in the left atrium, especially around the fossa ovalis, and their recurrence rate is minimal.
1) The other subgroup of familial myxomas mainly affects young people, and female predominance is not as marked. These cases are part of an autosomal-dominant disease known as Carney Complex Syndrome.
2)11)
In our series, the mean age at presentation was the fifth decade of life, and the slight predominance of women affected is in agreement with other published series.
5)10)12)13)14)15)
As imaging methods have improved, in vivo diagnosis of myxomas has increased. In our study, 17% of cases were detected during a routine echo exam performed for other reasons or to evaluate patients with cardiovascular risk factors; this incidental diagnosis rate is similar to those of other series.
12) These tumors were smaller than those found in symptomatic patients. Selkane et al.
13) recently showed that smaller tumors were resected in patients who were older and had more comorbidities, probably because such patients are evaluated more thoroughly for other specific health problems.
Although histologically benign, these tumors can be lethal due to their location in the circulatory system. The predominant site of tumor implantation is the fossa ovalis of the septum in the left atrium, similar to that described in other local
6)7) and international studies
5)10)12)13)14)15); the frequency of valvular and biatrial tumors were also similar to reports in other studies.
Although clinical manifestations vary among studies, the classic presentation comprises a triad of symptoms (“myxoma triad”), which includes embolic and obstructive phenomena and constitutional symptoms. In our study, as in the study by Pinede et al.
12), obstructive symptoms prevailed. Dyspnea was the most frequent complaint, evidenced by a high percentage of patients with signs of clinical heart failure. In the echocardiogram, these patients had a larger tumor diameter, greater valve obstruction, and pulmonary hypertension. Constitutional symptoms occurred in one-fourth of our patients, predominantly in larger tumors located in the left atrium, as described previously.
12)15) The etiologies of constitutional manifestation are not fully understood. They may be related to embolization (facial edema, myalgia, arthralgia, and nocturnal hemoptysis), erythropoietin production (erythrocytosis), mechanical destruction of cells by abnormal blood flow across the tumor surface (thrombocytopenia, hemolytic anemia-particularly associated with calcified tumors),
2) or autocrine production of cytokines such as IL6 and IL8 (inflammatory and autoimmune responses) accompanied by activation of the complement cascade by circulating antibody–tumor–antigen complexes.
16)17)18)
Embolic manifestations are the most severe due to their morbidity and mortality; they are attributed to detachment of small friable tumor fragments. In our study, 76.5% of villous myxomas presented with embolic episodes, whereas no embolic events occurred in patients with smooth surface myxomas. All patients who had embolic events had tumors with villous appearance on echocardiography; no patients with smooth myxomas had embolic episodes, in concordance with previous series.
10)19) Wang et al.
5) also proved that small myxomas predict embolism. In our series, patients with embolic manifestations had a villous surface but there were no differences in tumor size, which could be explained by the lower number of patients in this study.
This highlights the importance of the echocardiogram in describing the morphologic characteristics of these tumors, which predicts patients at higher risk of having an embolic complication and those who may require surgery as soon as possible after diagnosis.
Heterogeneous intratumor appearance was not associated with cardiovascular events. Although histology showed a correlation with foci of calcification, it was not useful in predicting foci of necrosis or hemorrhage.
Since Craford
20) performed the first surgery in a patient with a left atrial myxoma in 1954, several surgical techniques and safe approaches have been developed. In our study, the right atrial and transseptal approaches were used in most cases, and the left atrial tumor was resected through the atrial septum followed by simple closure. Currently, this technique is performed through a median sternotomy on cardiopulmonary bypass with prior aortic and bicaval cannulation and moderate hypothermia. Because it is safe and has very low mortality, it is the technique used most often.
5)12)14)21)
In our series, 12% of patients underwent valve repair. Some problems encountered relate to the condition of the valve apparatus, degree of concomitant mitral or tricuspid regurgitation, and the need for resection with an adequate safety margin, which might explain the need for valve repair.
Although the time between diagnosis and surgery varies among studies, we have no evidence regarding the urgency for intervention. However, the consensus is that elective but urgent surgery should be performed within 48 to 72 hours.
Surgery is safe, and perioperative mortality is low. Although elderly patients with comorbidities were operated on, there were no deaths in the immediate postoperative period, and long-term survival was good. These findings differ from those for patients who were not operated on, who had the lowest rate of 10-year survival. This may confirm the need for surgery in these patients, regardless of their surgical risk.
Although there were no perioperative deaths, most patients had some complication. As with other local
6)7) and international
5)10)12)13)14)15) studies, arrhythmias were the most common complication. These were mainly supraventricular in origin and occurred in one-third of the patients.
Once the postoperative period is over, long-term prognosis is good, similar to that of normal subjects; this study showed an excellent 10-year survival rate of close to 90%.
Recurrence was quoted to be less than 5%,
5)14)22)23) and although the familial form exhibits higher recurrence rates, it may also have occurred in sporadic forms. The cause of recurrence was unclear, although it was apparently due to growth of a remnant tumor focus that was not identified in the first surgery. Alternative hypotheses involve other mechanisms, such as spontaneous or perioperative intracardiac implantation and multicenter growth. Close echocardiographic follow-up is indicated for all operated myxomas to ensure early diagnosis and intervention in case of recurrence. This strategy allowed identification of tumor recurrence in a patient who did not have evidence of the familial form. He had initially undergone resection of a villous left atrial myxoma followed by simple closure of the septal resection. The second time, myxoma resection required a septal repair with a pericardial patch.
Conclusions
Dyspnea, constitutional symptoms, and embolism are the most frequent clinical manifestations of cardiac myxomas. Clinical presentation relates to the ultrasound characteristics of the tumor. Smooth tumors are larger, occur with obstructive symptoms, and benefit from elective surgery, whereas villous myxomas entailed a high embolic risk and required urgent surgical treatment. Surgery is low-risk once the immediate postoperative period is over, and long-term survival is adequate with a low recurrence rate during follow-up.









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download