Abstract
Intramural hematoma of the duodenum is a relatively unusual complication associated with the endoscopic treatment of bleeding peptic ulcers. Intramural hematomas are typically resolved spontaneously with conservative treatment alone. We report a case of an intramural duodenal hematoma following endoscopic hemostasis with epinephrine injection therapy, which was associated with transient obstructive jaundice in a patient undergoing hemodialysis. The patient developed biliary sepsis due to obstruction of the common bile duct secondary to the huge hematoma. He was treated with fluoroscopy-guided drainage catheter insertion, which spontaneously resolved the biliary sepsis through conservative treatment in 6 weeks. Fluoroscopy-guided drainage may impact the treatment of intramural hematomas that involve life-threatening complications.
Intramural hematoma of the gastrointestinal tract is a relatively uncommon condition. It is mainly caused by blunt trauma, bleeding disorders, or anticoagulation therapy.1 Recently, reports of intramural hematoma have increased with the use of therapeutic endoscopy for gastro-duodenal peptic ulcer bleeding.12 The risk of complications associated with endoscopic intervention is very low and mainly includes aspiration pneumonia and gut perforation.3 Intramural duodenal hematoma is a rare complication after an endoscopic intervention, although patients susceptible to bleeding such as those with end-stage renal disease (ESRD), liver cirrhosis, or those receiving anticoagulant therapy are more susceptible.456 We report a case of an intramural hematoma occurring after the endoscopic treatment of a duodenal ulcer in a patient undergoing hemodialysis (HD), followed by a secondary hematoma-induced common bile duct obstruction.
A 45-year-old man presented to emergency room of CHA Bundang Medical Center, complaining of multiple episodes of melena in 1 day. The patient had been on maintenance HD three times per week 6 years ago. He had a history of undergoing the Hartmann operation to treat left colic artery bleeding and colonic perforation a year ago. He had not taken non-steroidal anti-inflammatory drugs, antiplatelet drugs, or anticoagulants before hospitalization. At presentation, his vital signs were normal, and a physical examination revealed anemic conjunctiva without icteric sclera. The relevant laboratory investigations were as follows; hemoglobin 8.1 g/dL, platelet count 242,000/mm3, white blood cell count 10,810/mm3, INR 1.04, serum total bilirubin 0.35 mg/dL, serum AST 10 IU/L, serum ALT 16 IU/L, ALP 134 IU/L, CRP 5.98 mg/dL, BUN 116.1 mg/dL, and serum creatinine 7.8 mg/dL.
An emergency esophago-gastro-duodenoscopy (EGD) was performed due to suspected active gastrointestinal bleeding. The endoscopy revealed a deep ulcer with exposed vessel at the duodenal bulb (Fig. 1A). A total of 3 mL of 0.2% epinephrine injection, with subsequent hemoclipping, was administered to control the continuous oozing of blood from the exposed vessel. Moreover, 2 mL of fibrin glue injection was administered to treat the active bleeding from the duodenal ulcer (Fig. 1B). He was conservatively treated with an intravenous proton pump inhibitor.
On the second day after the endoscopic treatment, he complained of abdominal pain, a palpable mass and jaundice. The mass was palpated about 15 cm from the right upper quadrant site. The serum total bilirubin level had increased from 0.35 mg/dL, on admission, to 12.54 mg/dL, with elevations of the serum AST to 45 IU/L and ALT to 95 IU/L. An emergency abdominal CT scan revealed a common bile duct obstruction by a large hematoma of up to 20 cm in the submucosal area in the second to fourth portions of the duodenum (Fig. 2). He was conservatively managed with an intravenous proton pump inhibitor, continuous nasogastric suction, and total parenteral nutrition. For ESRD, the patient was administered heparin-free HD thrice weekly.
The next day, the patient developed fever (38.5℃). His pulse rate, respiratory rate, and blood pressure were 114/min, 18/min, and 90/60 mmHg, respectively. His white blood cell count increased to 16,720/mm3, and his CRP level was elevated to 19.87 mg/dL. Serum total bilirubin and serum amylase levels increased to 13.85 mg/dL and 511 IU/L, respectively. Sepsis was diagnosed due to the systemic inflammatory response syndrome and elevated infection markers. Empirical treatment with intravenous cefoperazone-sulbactam and metronidazole was started after two blood cultures. After fluid resuscitation, we attempted fluoroscopy-guided drainage catheter insertion because the patient was in biliary sepsis. An 8-Fr pigtail catheter was inserted through the right transhepatic access under a fluoroscopy guide and 170 mL of old blood was drained (Fig. 3). Blood culture and aspirate culture showed no growth. Six days after catheter insertion, follow-up abdominal CT scans revealed a decrease in the size of the intramural hematoma with an improvement of the common bile duct obstruction. However, oculated fluid collection was markedly increased in the right abdomen due to fistula formation leading to the retroperitoneum from the duodenal submucosal hematoma (Fig. 4).
Total parenteral nutrition was discontinued on the 24th day and the patient was slowly and carefully given liquids and then a soft meal. After 4 weeks of catheter drainage, follow-up abdominal CT findings showed progressive resolution of the hematoma (Fig. 5). When the amount of daily drainage was less than 5 mL and serum total bilirubin level was decreased to 4.76 mg/dL, the drainage catheter was removed. On the follow-up at 8 weeks, a repeat EGD revealed a healed duodenal ulcer with no evidence of hematoma (Fig. 6).
Intramural hematoma of the duodenum is a rare complication of endoscopic treatment. It is typically known to occur secondary to blunt abdominal trauma in children and young adults.12 However, this condition can occur in adults with underlying diseases that are susceptible to bleeding (e.g., liver cirrhosis, ESRD, leukemia, and any disease requiring anti-coagulation treatment), even with minimal trauma such as an endoscopic procedure.2345 Intramural hematomas are also reported in healthy patients, without risk factors, after endoscopic biopsies.7 In our case, the coagulation parameters and the platelet count were within the reference range. However, he had been on HD for a long time and the occurrence of hematoma in ESRD patients is well known.
In previously reported cases of intramural hematoma, most patients showed hemoperitoneum, pancreatitis, and partial or complete bowel obstruction with hematoma.1245678 Our case is rarer because the common bile duct obstruction occurred secondary to the large intramural duodenal hematoma. The development of fever, leukocytosis, and hyperbilirubinemia after repeated endoscopic procedures suggested the presence of biliary sepsis due to complications associated with the intramural hematoma. However, the patient had been diagnosed with ESRD and was receiving HD. And he had undergone a surgery for a large bowel perforation a year ago. It was therefore difficult to suggest surgical intervention for intramural hematoma removal. In addition, this patient had biliary sepsis caused by the common bile duct obstruction with jaundice, which also increased the risks of surgical intervention. Therefore, we tried hematoma decompression through fluoroscopy-guided drainage catheter insertion despite the risk of catheter bleeding. The common bile duct obstruction was resolved as the intramural hematoma reduced in size.
The mechanism of hematoma formation due to endoscopic intervention is not yet clear. However, various factors involving iatrogenic trauma (e.g., needle, scope, instrument), as well as underlying conditions, may contribute to its development. First, rupture of the arterial blood vessel by needle injection may result in the subsequent formation of a hematoma in predisposed individuals because the gastro-duodenal wall is fixed by the retroperitoneal location and the rich submucosal vascular supply.8 Second, it is possible that the mucosa of the gastrointestinal tract wall may have been excessively pulled up by forceps during the endoscopic treatment procedure, which might damage the submucosal blood vessels. Zinelis et al.7 suggested that a significant amount of mucosal tissue can be pulled out and away from the fixed wall of the mucosa underneath, with more than 3 cm from the endoscope tip during the biopsy forceps procedure. In our case, we did not use biopsy forceps; however, we presume that the hematoma occurred due to injection during the endoscopic hemostasis.
The diagnosis of intramural duodenal hematoma is based on symptoms of bowel obstruction and a decrease in hemoglobin level without overt signs of bleeding. An enhanced abdominal CT serves as the best imaging tool for the diagnosis.9 In principle, the management of this condition is typically conservative treatments. Because of the rich blood supply of the duodenal wall, the hematoma is anticipated to be absorbed with time. Conservative treatments include total parenteral nutrition and electrolyte replacement, nasogastric tube decompression and careful observation.
On the other hand, surgical treatment may be considered when there is a septic condition, with obstruction secondary to the hematoma, such as the current case. In addition, aggressive treatment should be considered when there is no evidence of partial resolution after conservative treatment, or in cases of perforation or peritonitis, which increases the size of the hematoma.78 Recently, several new therapeutic strategies have been reported. Kwon et al.6 reported a case of intramural hematoma of the duodenum, which was treated with percutaneous drainage and embolization of the bleeding focus, similar to our case. They also recommended endoscopic incision and drainage for treatment.8 Aizawa et al.10 reported that a patient with a duodenal hematoma was treated with ultrasound-guided drainage and balloon dilatation.
In conclusion, an intramural duodenal hematoma can occur following a common endoscopic treatment in patients susceptible to bleeding. Therefore, the possibility of an intramural hematoma should be considered in any patient with symptoms such as gastrointestinal obstruction or pancreatitis, abdominal pain, nausea, vomiting, and jaundice following an endoscopic treatment. Noninvasive diagnostic tools such as EGD or abdominal CT scans may help in early diagnosis of intramural hematomas. We suggest that fluoroscopy-guided drainage may also play a role in the treatment of intramural hematomas, if conservative treatment is limited.
Figures and Tables
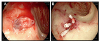 | Fig. 1Endoscopic findings. (A) The esophago-gastro-duodenoscopy on admission revealed a deep ulcer and an exposed vessel on the base was noted at the duodenal bulb. (B) An epinephrine injection (2 mL, 1 mL) was administered to control the blood oozing from the vessel. Complete hemostasis was acquired with four hemoclippings and 2 mL of fibrin glue injection. |
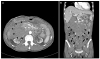 | Fig. 2On the 2nd hospital day, the contrast-enhanced abdominal computed tomography scan showed a huge intramural hematoma in the 2nd to 4th portions of the duodenum with compression of the common bile duct (black arrows, intramural hematoma; white arrows, common bile duct). (A) Axial image. (B) Coronal image. |
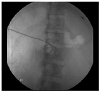 | Fig. 3Fluoroscopic finding. An 8-Fr pigtail catheter was inserted through the right transhepatic access to the duodenal submucosal hematoma. |
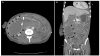 | Fig. 4Six days after catheter insertion, the follow-up abdominal computed tomography showed partial regression of the intramural hematoma and mild improvement of the common bile duct obstruction. A marked increase in loculated fluid collection was shown in the right abdomen with fistula formation leading to the retroperitoneum from the duodenal submucosal hematoma (black arrows, hematoma fluid loculation; arrowheads, fistula; white arrows, common bile duct). (A) Axial image. (B) Coronal image. |
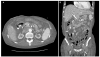 | Fig. 5After 4 weeks of catheter drainage, the follow-up abdominal computed tomography showed near resorption of the hematoma in the submucosal area of the duodenum and decreased loculated fluid collection in the right abdomen (black arrow, resolved intramural hematoma; white arrows, decreased loculated fluid). (A) Axial image. (B) Coronal image. |
References
1. Rohrer B, Schreiner J, Lehnert P, Waldner H, Heldwein W. Gastrointestinal intramural hematoma, a complication of endoscopic injection methods for bleeding peptic ulcers: a case series. Endoscopy. 1994; 26:617–621.


2. Dhawan V, Mohamed A, Fedorak RN. Gastric intramural hematoma: a case report and literature review. Can J Gastroenterol. 2009; 23:19–22.



3. Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009; 7:33–47. quiz 1–2.


4. Chung S, Park CW, Chung HW, Shin SJ, Chang YS. Intramural duodenal hematoma and hemoperitoneum after endoscopic treatment in a patient with chronic renal failure on hemodialysis: a case report. Cases J. 2009; 2:9083.



5. Sugai K, Kajiwara E, Mochizuki Y, et al. Intramural duodenal hematoma after endoscopic therapy for a bleeding duodenal ulcer in a patient with liver cirrhosis. Intern Med. 2005; 44:954–957.

6. Kwon CI, Choi KH, Ko EH, et al. A case of duodenal intramural hematoma treated by percutaneous external drainage. Korean J Gastroenterol. 2007; 49:45–49.

7. Zinelis SA, Hershenson LM, Ennis MF, Boller M, Ismail-Beigi F. Intramural duodenal hematoma following upper gastrointestinal endoscopic biopsy. Dig Dis Sci. 1989; 34:289–291.


8. Kwon CI, Ko KH, Kim HY, et al. Bowel obstruction caused by an intramural duodenal hematoma: a case report of endoscopic incision and drainage. J Korean Med Sci. 2009; 24:179–183.



9. Plojoux O, Hauser H, Wettstein P. Computed tomography of intramural hematoma of the small intestine: a report of 3 cases. Radiology. 1982; 144:559–561.


10. Aizawa K, Tokuyama H, Yonezawa T, et al. A case of traumatic intramural hematoma of the duodenum effectively treated with ultrasonically guided aspiration drainage and endoscopic balloon catheter dilation. Gastroenterol Jpn. 1991; 26:218–223.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download