Paired box 8 (PAX8) is a transcription factor, which in humans is encoded by the PAX8 gene, located on the chromosome 2, cytoband q14.1.1 It belongs to the paired box protein family, consisting of nine members from PAX1 to PAX9.1 This family of transcription factors is so called because all the members share the same N-terminal paired box domain composed of about 120 amino acids. The PAX proteins are further subdivided into four groups on the basis of the presence (or absence) in their molecular structure of an octopeptide and of a paired-type homeobox at the C-terminal domain: PAX8 is placed in the second group together with PAX5 and PAX2.2
PAX8 is a master regulator in thyroid development; moreover, it plays an important role in the organogenesis of the thymus, kidneys, Müllerian system, eyes, and brain.34567
Several immunohistochemical studies have demonstrated the importance of PAX8 as immunomarker for different epithelial neoplasms arising in the thyroid and parathyroid gland, thymus, female and male genital tract or kidneys, including the Wilms' tumor.8910111213
Some authors have also reported PAX8 expression in pancreatic islet cells and in neuroendocrine tumors of the pancreas, as well as in carcinoids arising in the duodenum, rectum and stomach.1415
Consequently, the pathologists exploit PAX8 immunostaining to differentiate primary and metastatic PAX8-positive tumors from PAX8-negative ones.912
As mentioned above, PAX5 is inserted in the same group as PAX8, however, unlike PAX8, PAX5 is encoded by a gene located on chromosome 9, cytoband p13.2, and it plays a significant role in early B-cell differentiation as well as in neural development and spermatogenesis.31617
PAX5 immunostaining is used by pathologists in the diagnosis of most B-cell non-Hodgkin lymphomas/leukemias, Hodgkin lymphomas, where it faintly depicts Reed-Sternberg and Hodgkin cells, and in the differential diagnosis between plasmacytoma (PAX5-negative) and lymphoplasmacytic lymphoma/marginal zone lymphoma with plasmacytoid differentiation (PAX5-positive).16171819
As it is well known, a secondary follicle, which arises from a primary follicle after an antigenic stimulation of B cells, is provided with a germinal center and a mantle zone. Externally to the mantle zone there is the marginal zone, which is difficult to distinguish, except when it is expanded, as in the course of benignant or lymphomatous proliferations.20212223
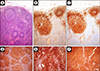
Literature has reported that PAX8 stains B lymphocytes and B-cell lymphomas.242526 To prove the validity of this statement, we have at first immunohistochemically evaluated the PAX5 and PAX8 expression in 50 consecutive normal lymph nodes removed for staging purposes in as many patients affected by epithelial malignancies. In all the tested cases, PAX8 was labeled at the germinal center, the mantle, and the marginal zone, exactly as PAX5 (Fig. 1). Subsequently, we have enrolled 25 cases of germinal center-derived lymphomas, 25 cases of mantle cell lymphoma and 25 cases of marginal zone lymphomas, coming from patients of any age, gender, or ethnicity, all resulted immunohistochemically positive for PAX5 and PAX8, as deriving from mature B cells (Fig. 1).
Since PAX8 is not involved in the process of B-lymphocyte commitment maintaining the same immunohistochemical profile of PAX5, many authors have wondered if that of PAX8 was a real positive. For example, Moretti and colleagues have tested PAX8 and PAX5 in reactive lymph nodes, in diffuse large B-cell lymphomas, and in Hodgkin lymphomas using for PAX5 a monoclonal antibody, and for PAX8 both a N-terminal polyclonal antibody (pPAX8), and a C-terminal monoclonal antibody (mPAX8).27 The results were the same for pPAX8 and PAX5, that were both positive in all lymphomas and reactive lymph nodes, while mPAX8 was negative in all the tested cases.27 In a similar manner, Morgan et al.28 investigated pPAX8, mPAX8 and PAX5 in a cohort of B-cell lymphomas, obtaining the same expression profile for pPAX8 and PAX5. In conclusion, we can state that PAX8 and PAX5 immunohistochemistry are overlapping in the routine diagnostic practice and, therefore, PAX8 positivity in lymphomas should not be surprising. On the other hand, this evidence should be kept in mind by the pathologists in order to avoid diagnostic pitfalls and mistakes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download