This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Objectives
Aortic valve replacement (AVR) is the treatment of choice in severe symptomatic aortic stenosis (AS) patients. However, a substantial number of elderly patients refuse AVR and treated medically. We investigated their long-term prognosis.
Methods
From January 2005 to December 2016, we analyzed elderly patients with severe symptomatic AS who refused to have AVR.
Results
After screening of total 534 patients, we analyzed total 180 severe symptomatic AS patients (78±7 years old, 96 males). Hypertension was the most common cardiovascular risk factor (72%) and the most common symptom was dyspnea (66%). Calculated aortic stenosis area was 0.73±0.20 cm2 and mean left ventricular ejection fraction (LVEF) was 57.8±12.2%. Total 102 patients died during follow-up period (39.1±31.0 months). One-, 3-, and 5-year all-cause mortality rate was 21.1±3.0%, 43.1±3.8%, and 56.5±4.2%, respectively. Of them, 87 died from cardiac causes, and 1-, 3-, and 5-year cardiac mortality rate was 18.0±2.9%, 38.2±3.8%, and 50.7±4.3%, respectively. Their all-cause mortality and cardiac mortality were significantly higher than those of controls. Univariate analysis showed that age, anemia, LVEF, and Log N-terminal pro B-type natriuretic peptide (NT-proBNP) were significant parameters in all-cause mortality (p<0.001, p=0.001, p=0.039, and p=0.047, respectively) and in cardiac mortality (p<0.001, p<0.001, p=0.046, and p=0.026, respectively). Multivariate analysis showed that age and anemia were significant prognostic factors for cardiac and all-cause mortality.
Conclusions
In elderly severe symptomatic AS patients who treated medically, their 1-, 3- and 5-year all-cause mortality rate was 21.1±3.0%, 43.1±3.8%, and 56.5±4.2%, respectively. Age and anemia were significant prognostic factors for cardiac and all-cause mortality.
Keywords: Aortic valve stenosis, Survival, Prognosis, Drug therapy
INTRODUCTION
Aortic stenosis (AS) is the most common valvular heart disease especially in elderly patients and its incidence is growing with the increase of elderly population.
1) In the US population-based study in 2005, the extrapolated prevalence of aortic valve (AV) disease was 1.8% (approximately 5.2 million people) and the prevalence was 10.7% in persons aged ≥65 years.
2)
In the previous studies, symptom onset is major determinant of poor clinical outcomes in patients with severe AS.
3)4) Usually surgical or percutaneous aortic valve replacement (AVR) is the treatment of choice in patients with symptomatic AS. However, there are substantial portion of symptomatic AS patients who do not receive AVR for various reasons especially in elderly patients. In Korea, prevalence of non-rheumatic degenerative AV disease was 72 patients per 100,000 persons.
5) Because AV disease increases with aging, increasing aged-population would be associated with increase of its incidence in Korea. Moreover, there can be a substantial number of symptomatic elderly AS patients who requiring AVR. However, the transcatheter aortic valve replacement (TAVR) has not been covered by the National Health Insurance Service in Korea, and TAVR has been performed in limited cases. Thus, there should be many elderly patients with symptomatic AS who treated with medically. However, we have no long-term prognosis data about these patients. So, we investigated long-term clinical outcomes of elderly patients with symptomatic severe AS who refused taking AVR.
METHODS
This is a retrospective observational study in three tertiary referral hospitals in Korea. We screened all severe AS from January 2005 to December 2016. We included all elderly patients (age over 65 years old) with symptomatic AS who refused undertaking AVR and treated conservatively. We excluded patients without symptoms, patients who underwent surgical or percutaneous AVR within 6 months interval from the diagnosis, patients with concomitant moderate to severe valvular diseases other than AS and patients with other obvious causes of developing symptoms other than AS.
Baseline clinical data were collected from their medical records including age, sex, history of hypertension, diabetes and presence of coronary artery diseases (CADs). Hypertension was defined with the use of antihypertensive medications or average office blood pressure >140–90 mmHg. Diabetes was defined as the use of hypoglycemic agents or fasting glucose level >126 mg/dL and/or 200 mg/dL at 2 hours after meal. CAD was identified with a history of myocardial infarction, percutaneous coronary intervention, or angiographically documented coronary artery stenosis. The presence of heart failure (HF) was defined with signs or symptoms of HF with either pulmonary congestion, or objective findings of left ventricular (LV) systolic dysfunction or structural heart disease. LV systolic dysfunction was defined as left ventricular ejection fraction (LVEF) less than 50%, and structural heart disease defined as previous diagnosis of ischemic heart disease, dilated cardiomyopathy, valve dysfunction (mild valvular stenosis and regurgitation were not included), or primary myocardial structural disease. Patients were classified into 1 category, including dyspnea, angina, syncope, and HF, according to their initial symptom. To compare the natural history of our study group, we included normal controls who had national health examination and included age- and sex-matched controls 5 times more than our study population.
All echocardiographic examinations were done with the standardized guidelines using commercially available ultrasound systems by the American Society of Echocardiography.
6) The standard echocardiographic examinations included M-mode, 2-dimensional and Doppler measurements. We measured peak trans-AV velocity by continuous-wave (CW) Doppler using multiple imaging windows including an apical 5 chamber, apical 3 chamber, right parasternal and suprasternal views. Mean trans-AV pressure gradient (PG) was calculated using the Bernoulli equation with tracing of the CW Doppler tracing of AV. Aortic stenosis area (AVA) was calculated by the continuity equation. LVEF was measured by the biplane Simpson's method with apical 4-chamber and apical 2-chamber views. Severe AS was defined with calcified AV with peak AV velocity ≥4.0 m/sec, mean trans-AV PG ≥40 mmHg or AVA <1.0 cm
2 by the continuity equation. We classified our patients as their LVEF and mean transvalvular gradient. Low LVEF was defined as LVEF less than 50% and a low mean transvalvular gradient was regarded as gradient less than 40 mmHg. Patients were categorized into 4 groups; high gradient severe AS with preserved LVEF (HGpEF), high gradient severe AS with reduced LVEF (HGrEF), low gradient severe AS with preserved LVEF (LGpEF), and low gradient severe AS with reduced LVEF (LGrEF).
We checked all-cause mortality with their medical records in patients with regular clinical follow up and data from the Ministry of Public Administration and Security in patients without regular follow-up. The study complied with the Declaration of Helsinki principles. The study protocol was approved by the Institutional Review Board (IRB) of each hospital (2016-12-041). The IRBs waved the need for a written informed consent from the study patients.
Quantitative data are expressed as mean±standard deviation and categorical variables are summarized as numbers and frequencies. For comparison of 2 groups according to the presence of all-cause mortality, we used the Student's t-test for continuous variables and the χ2 test for categorical variables. Survival analysis was done using the Kaplan-Meier method, and time to clinical events was analyzed using the multivariate Cox-proportional hazard analysis. We performed multivariate analysis with all variables as covariates found to be statistically significant (p<0.05) in the univariate analysis or variables known to be clinically important, excluding those with multicollinearity with others. The data were analyzed using SPSS version 22 (IBM Corp., Armonk, NY, USA) and MedCalc version 18 (MedCalc Software, Ostend, Belgium). A 2-sided p value of <0.05 was considered statistically significant.
RESULTS
Initially we screened a total of 534 patients (age over 65 years old) with severe AS. We excluded 218 patients without symptoms, 103 patients who underwent surgical or percutaneous AVR, 27 patients with other concomitant significant valvular heart disease and 6 patients with obvious causes of symptoms other than AS. Thus, we analyzed a total of 180 patients with severe symptomatic AS. The scheme of this study population was summarized in the
Figure 1.
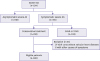 | Figure 1
Scheme of study population.
AS = aortic stenosis; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.

|
Baseline characteristics were summarized in the
Table 1. Mean age was 78±7 years old and 96 were male (53%). Hypertension was the most common cardiovascular risk factor (72%), and anemia and atrial fibrillation were in 92 patients (51%) and 23 patients (13%), respectively. Calculated AVA was 0.73±0.20cm
2, peak trans-AV Vmax was 4.6±0.8 m/s and mean trans-AV PG was 50.7±18.5 mmHg. Thirty-three patients (18%) had LVEF less than 50%.
Table 1
Baseline clinical and echocardiographic characteristics

|
Variables |
All (n=180) |
|
Age (years) |
78±7 |
|
Male sex (%) |
96 (53) |
|
Concomitant diseases |
|
|
Hypertension (%) |
129 (72) |
|
Diabetes (%) |
48 (27) |
|
Chronic kidney disease (%) |
13 (7) |
|
Dialysis (%) |
11 (6) |
|
Anemia (%) |
92 (51) |
|
Ischemic heart disease (%) |
12 (7) |
|
Previous myocardial infarction (%) |
7 (4) |
|
Atrial fibrillation (%) |
23 (13) |
|
Peripheral artery disease (%) |
3 (2) |
|
Prior stroke (%) |
12 (7) |
|
Initial symptom |
|
|
Dyspnea (%) |
119 (66) |
|
|
NYHA I |
18 (10) |
|
|
NYHA II |
57 (32) |
|
|
NYHA III |
23 (13) |
|
|
NYHA IV |
21 (12) |
|
Angina pectoris (%) |
33 (18) |
|
Syncope (%) |
11 (6) |
|
Congestive HF (%) |
17 (9) |
|
Laboratory findings |
|
|
Hemoglobin (g/dL) |
11.7±2.1 |
|
Creatinine (g/dL) |
1.3±1.4 |
|
NT-proBNP (pg/mL) |
3,035±5,009 |
|
Echocardiographic findings |
|
|
LVEF (%) |
57.8±12.2 |
|
LVEF <50% (%) |
35 (18) |
|
Peak trans-AV velocity (m/s) |
4.6±0.8 |
|
Peak trans-AV PG (mmHg) |
87.7±29.5 |
|
Mean trans-AV gradient (mm Hg) |
50.7±18.5 |
|
AVA (cm2) |
0.73±0.20 |
|
Etiology of AS |
|
|
Degenerative, tricuspid (%) |
170 (93) |
|
Degenerative, bicuspid (%) |
7 (4) |
|
Rheumatic (%) |
3 (2) |

A total of 102 patients died during the follow-up period of 39.1±31.0 months. One-, 3-, and 5-year mortality rates were 21.1±3.0%, 43.1±3.8%, and 56.5±4.2%, respectively (
Figure 2A). All-cause mortality was much higher than that of normal controls; 1-, 3-, and 5-year mortality rates were 1.3±0.4%, 11.4±1.0%, and 25.7±1.4%, respectively (p<0.001, p=0.010, and p=0.014, respectively). Of them, 87 died from cardiac causes and 15 had non-cardiac causes of death (pneumonia and chronic obstructive pulmonary disease in 8 patients, cancer in 3 patients, sepsis in 2 patients, chronic kidney disease in 1 patient, and fracture in 1 patient). One-, 3-, and 5-year cardiac mortality rate was 18.0±2.9%, 38.2±3.8%, and 50.7±4.3%, respectively (
Figure 2B). Cardiac mortality was much higher than that of normal controls; 1-, 3-, and 5-year mortality rate was 0.2±0.1%, 3.0±0.6%, and 6.4±0.8%, respectively (p=0.005, p=0.008, and p=0.010, respectively).
 | Figure 2
All-cause mortality and cardiac mortality. One-, 3- and 5-year mortality rate is 21.1±3.0%, 43.1±3.8% and 56.5±4.2%, respectively (A) All-cause mortality of our study population is much higher than that of normal controls (1-, 3-, and 5-year survival rate was 1.3±0.4%, 11.4±1.0%, and 25.7±1.4%, respectively (p<0.001, p=0.010, and p=0.014, respectively). One-, 3- and 5-year cardiac mortality rate is 18.0±2.9%, 38.2±3.8% and 50.7±4.3%, respectively (B). Which is much higher than that of normal controls (1-, 3-, and 5-year survival rate was 0.2±0.1%, 3.0±0.6%, and 6.4±0.8%, respectively (p=0.005, p=0.008, and p=0.010, respectively).
Sx AS = symptomatic aortic stenosis.

|
Among 4 presenting symptoms including dyspnea, angina, syncope and HF, dyspnea was the most common symptom (66%). When we analyzed mortality curve according to their initial symptoms, there was no difference among the groups in all-cause mortality (p=0.415,
Figure 3A) and cardiac mortality (p=0.767,
Figure 3B). However, in the dyspnea group, severely symptomatic patients (defined as New York Heart Association [NYHA] III–IV) had significantly higher rate of all-cause mortality (hazard ratio [HR], 2.41; 95% confidence interval [CI], 1.49–3.90; p<0.001) and cardiac mortality (HR, 2.59; 95% CI, 1.52–4.40; p<0.001) compared with minimally symptomatic patients (defined as NYHA I–II). All-cause mortality and cardiac mortality were observed in 65 (58%) and 57 (51%) in the HGpEF group, 13 (65%) and 10 (50%) in the HGrEF group, 18 (50%) and 15 (42%) in the LGpEF group, and 6 (50%) and 5 (42%) in the LGrEF group, respectively. In Cox proportional hazards analysis, there was no significant difference in all-cause mortality (p=0.545,
Figure 4A) and cardiac mortality (p=0.838,
Figure 4B) among the 4 groups.
 | Figure 3
All-cause mortality (A) and cardiac mortality (B) according to their initial presenting symptoms. There is no significant difference among groups in all-cause mortality (p=0.415) and cardiac mortality (p=0.767).
HF = heart failure.

|
 | Figure 4
All-cause mortality (A) and cardiac mortality (B) according to PG and LVEF, such as HGpEF, HGrEF, LGpEF, and LGrEF. There is no significant difference among the 4 groups in all-cause mortality and cardiac mortality.
HGpEF = high gradient severe aortic stenosis with preserved left ventricular ejection fraction; HGrEF = high gradient severe aortic stenosis with reduced left ventricular ejection fraction; LGpEF = low gradient severe aortic stenosis with preserved left ventricular ejection fraction; LGrEF = low gradient severe aortic stenosis with reduced left ventricular ejection fraction; LVEF = left ventricular ejection fraction; PG = pressure gradient.

|
Univariate Cox proportional hazard analysis (
Table 2), age, anemia, LVEF and log N-terminal pro B-type natriuretic peptide (NT-proBNP) were significant parameters in the prediction of all-cause mortality (p<0.001, p<0.001, p=0.039, and p=0.047, respectively) and cardiac mortality (p<0.001, p=0.001, p=0.046, and p=0.026, respectively). AVA had tendency to have clinical significance in the prediction of cardiac mortality (HR, 0.35; 95% CI, 1.12–1.02; p=0.054). After multivariable adjustment (
Table 2), age and anemia were significant parameters in the prediction of all-cause mortality (HR, 1.04; 95% CI, 1.01–1.07; p=0.017 and HR, 1.89; 95% CI, 1.21–2.93; p=0.005) and cardiac mortality (HR, 1.05; 95% CI, 1.01–1.09; p=0.008 and HR, 1.93; 95% CI, 1.19–3.12;p=0.0074, respectively.
Table 2
Univariate and multivariate analysis in the prediction of all-cause mortality and cardiac mortality

|
Univariate |
Multivariate |
|
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
All-cause mortality |
|
|
|
|
|
Age (years) |
1.05 (1.02–1.08) |
<0.001 |
1.04 (1.01–1.07) |
0.017 |
|
Male sex |
0.75 (0.51–1.12) |
0.160 |
0.67 (0.43–1.04) |
0.074 |
|
Hypertension |
1.07 (0.70–1.648) |
0.753 |
- |
- |
|
Diabetes |
0.82 (0.51–1.300) |
0.393 |
- |
- |
|
Chronic kidney disease |
1.52 (0.74–3.138) |
0.258 |
- |
- |
|
Atrial fibrillation |
1.45 (0.85–2.476) |
0.175 |
- |
- |
|
Ischemic heart disease |
1.26 (0.61–2.590) |
0.534 |
- |
- |
|
LVEF (%) |
0.98 (0.97–0.999) |
0.039 |
0.98 (0.96–1.00) |
0.059 |
|
Trans-AV Vmax (m/s) |
0.96 (0.73–1.256) |
0.749 |
1.04 (0.73–1.48) |
0.847 |
|
AVA (cm2) |
0.40 (0.15–1.089) |
0.073 |
0.57 (0.15–2.21) |
0.415 |
|
Anemia |
2.08 (1.37–3.17) |
0.001 |
1.89 (1.21–2.93) |
0.005 |
|
Creatinine (mg/dL) |
1.10 (0.98–1.231) |
0.119 |
- |
- |
|
Log (NT-proBNP) |
1.49 (1.01–2.20) |
0.047 |
- |
- |
|
Cardiac mortality |
|
|
|
|
|
Age (year) |
1.06 (1.03–1.09) |
<0.001 |
1.05 (1.01–1.09) |
0.008 |
|
Male (sex) |
0.84 (0.55–1.29) |
0.429 |
0.70 (0.44–1.13) |
0.142 |
|
Hypertension |
1.05 (0.66–1.67) |
0.833 |
- |
- |
|
Diabetes |
0.84 (0.51–1.38) |
0.480 |
- |
- |
|
Chronic kidney disease |
1.53 (0.70–3.32) |
0.284 |
- |
- |
|
Atrial fibrillation |
1.36 (0.75–2.46) |
0.307 |
- |
- |
|
Ischemic heart disease |
1.30 (0.60–2.83) |
0.501 |
- |
- |
|
LVEF (%) |
0.98 (0.97–1.00) |
0.046 |
0.98 (0.96–1.00) |
0.054 |
|
Trans-AV Vmax (m/s) |
0.98 (0.73–1.31) |
0.884 |
1.03 (0.70–1.52) |
0.866 |
|
AVA (cm2) |
0.35 (0.12–1.02) |
0.054 |
0.54 (0.12–2.36) |
0.411 |
|
Anemia |
2.15 (1.36–3.39) |
0.001 |
1.93 (1.19–3.12) |
0.007 |
|
Creatinine (mg/dL) |
1.10 (0.98–1.23) |
0.119 |
- |
- |
|
Log (NT-proBNP) |
1.65 (1.06–2.55) |
0.026 |
- |
- |

DISCUSSION
In this study, we showed 57% of our elderly study population died during the follow-up period. Their 1-, 3-, and 5-year all-cause mortality rate was 21.1±3.0%, 43.1±3.8%, and 56.5±4.2%, respectively, and 1-, 3-, and 5-year cardiac mortality rate was 18.0±2.9%, 38.2±3.8%, and 50.7±4.3%, respectively. Age and anemia were significant prognostic factors in the prediction of all-cause mortality and cardiac mortality in the multivariate analysis.
Traditionally, the presence of symptoms was the most important prognostic factor. When severe AS patients present even mild symptoms, their survivals are poor unless the obstruction is relieved. The approximate survival interval from the onset of symptoms to the time of death is about 2 years in patients with HF, 3 years in patients with syncope and 5 years in those with angina.
3) However, these data came from very old retrospective study. Thus, we need more recent study data.
In our study, 1-year all-cause mortality was 21.1±3.0%. This result seems similar to that of recent studies. Bach et al.
7) reported their data of severe symptomatic AS patients from 3 large tertiary care centers. They reported 1-year mortality rate was 33.5±4.7% in unoperated severe symptomatic AS patients. However, in the Placement of AoRTic TraNscathetER Valve Trial (PARTNER) trial, 1-year all-cause mortality rate in the medically treated group was 50.9% and 2-year mortality rate was 68.0%.
8)9) These were significantly higher than that of patients receiving TAVR treatment (HR, 0.58; 95% CI, 0.43–0.78; p<0.001), Their mortality rates look higher than those of our study. However, age of the study population in our study was much lower than that of the PARTNER trial (mean difference, 5.2 years; p<0.001). Age is a well-known bad prognostic factor. In 1 study, age over 80 was strongly associated with perioperative mortality even in patients underwent surgical AVR.
7) Beside age, the incidence of CAD was much prevalent in the PARTNER trial than that of ours. Moreover, proportion of obese or overweight and ethnic difference might influence the survivals.
10)
Traditionally, the presence of HF symptoms has been regarded as the poorest prognostic factor in the symptomatic AS patients. However, there was no significant difference among symptoms in our study. Although there could be a bias and limitations in the definition of symptoms, our study can emphasize that patients with even mild symptoms had poor long-term prognosis.
Although peak trans-AV velocity did not have statistical significance, LVEF was another prognostic marker in our study. When the LV begins to fail, the cardiac output and trans-AV gradient are getting decreased. In our study, 48 (27%) patients had a low gradient severe AS, and these patients had a high rate of clinical events during follow-up; 50% and 41.7% of patients had experienced all-cause death or cardiac death, respectively. For these reasons, trans-AV velocity and PG did not have clinical significance for all-cause mortality or cardiac mortality. However, AVA had tendency to predict cardiac mortality in our study population. The presence of anemia and NT-proBNP levels were well established prognostic factors of severe AS. Because anemia results in decreased tissue perfusion, increased LV hypertrophy and interstitial fibrosis. Similarly, AS results in LV hypertrophy, increased systemic vascular resistance, and reduced cardiac output. Thus, the concomitant presence of anemia and severe AS will lead to reduced myocardial perfusion and increased oxygen demand, causing myocardial ischemia and potentially exacerbate HF, increased all-cause mortality.
11)
The risk of sudden death is high in patients with symptomatic severe AS. In our study, 66 of 87 (75.9%) patients might die suddenly. Also, there are many recurrent hospitalizations for decompensated HF and/or angina pectoris in severe symptomatic AS patients who refused surgical or percutaneous intervention.
12) Clark et al.
12) reported their data from Medicare in the US. They included 2,150 patients with medically managed severe AS. The mean age of their cohort was 82 years, estimated European System for Cardiac Operative Risk Evaluation score was 17%. During 5-year follow-up, overall mortality rate was 88.4% with a mean survival time was 1.8 years. During this time, patients had an average of 4.4 hospital admissions and the total 5-year costs were $63,844 per patients. Although there are several different medical systems, the pattern of recurrent hospitalization should be similar in Korea. Recent study showed the clinical outcomes of TAVR in Korean registry and in Asian population, including Koreans were favorable compared with those of studies from western countries.
13)14) Moreover, there are favorable result of suture-less AVR in elderly patients.
15) Thus, physicians should pay attention to undergoing prompt treatment to relieve the PG such as TAVR to prevent significant consumption of health care resources associated with recurrent hospitalizations of these patients.
This study has several limitations mainly came from the nature of study. This study is a retrospective study with review of medical records and data from the Ministry of Public Administration and Security. Thus, there might be a selection bias, and we had missing data about hospitalization of them. Second, the prevalence of CAD might be underestimated in this study because only 76 (42.2%) of our patients underwent coronary evaluation. Third, we did not include outcomes of patients who underwent surgical or percutaneous AVR. Moreover, there is no data about the medical cost of patients undergo surgical AVR or TAVR versus medical treatment only in our patients. If with these data, the comparison of survival will be much better. To overcome these limitations, well designed registry data should be needed in near future.
In conclusion, in elderly patients who treated medically, their 1-, 3-, and 5-year all-cause mortality rate was 21.1±3.0%, 43.1±3.8%, and 56.5±4.2%, respectively and 1-, 3-, and 5-year cardiac mortality rate was 18.0±2.9%, 38.2±3.8%, and 50.7±4.3%, respectively. The mortality rates were much higher than those without AS.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download