This article has been
cited by other articles in ScienceCentral.
Abstract
Background
To objectively investigate accommodative response to various refractive stimuli in subjects with normal accommodation.
Methods
This prospective, non-randomized clinical trial included 64 eyes of 32 subjects with a mean spherical equivalent −1.4 diopters (D). We evaluated changes in accommodative power, pupil diameter, astigmatic value, and axis when visual stimuli were applied to binocular, monocular (dominant eye, non-dominant eye, ipsilateral, and contralateral), and pinhole conditions. Visual stimuli were given at 0.25 D (4 m), 2 D (50 cm), 3 D (33 cm), and 4 D (25 cm) and accommodative response was evaluated using open view binocular autorefractor/keratometer.
Results
The accommodative response to binocular stimulus was 90.9% of the actual refractive stimulus, while that of the monocular stimulus was 84.6%. The binocular stimulus induced a smaller pupil diameter than did the monocular stimulus. There was no difference in accommodative response between the dominant eye and non-dominant eye or between ipsilateral and contralateral stimuli. As the refractive stimuli became stronger, the absolute astigmatic value increased and the direction of the astigmatism axis became more horizontal. Pinhole glasses required 10%–15% less accommodative power compared with the monocular condition.
Conclusion
Binocular stimuli enable more precise and effective accommodation than do monocular stimuli. Accommodative response is composed of 90% true accommodation and 10% pseudo-accommodation, and the refractive stimulus in one eye affects the contralateral eye to the same extent. This should be taken into account when developing guidelines for wearing smart glasses while driving, as visual stimulation is applied to only one eye, but far distance attention is constantly needed.
Keywords: Accommodation, Astigmatism, Pinhole, Pupil Diameter
INTRODUCTION
Accommodation is defined as optical power changes that occur to focus on an object at various distances (especially near) or maintain a clear image on the retina.
123 During accommodation, pupillary constriction occurs to increase the depth of focus
45 by narrowing incident rays and blocking aberrant rays
67 into the retina. The lens under control of the ciliary muscles becomes more biconvex to increase its refractive power. Convergence of the optic axis occurs to focus on near objects clearly.
8 The afferent limb, which recognizes stimuli from retinal ganglion cells to visual cortex, and the efferent limb, which connect the impulse from the visual cortex to the Edinger-Westphal nucleus of the mid-brain, comprise the accommodation reflex triad.
8910 This innervation mechanism explains the bilateral symmetrical accommodative response to accommodative stimuli as a yoked consensual response. Besides, unsighted eyes in cases of unilaterally blind eyes display similar accommodative responses to their fellow sighted eyes when accommodation stimuli are elicited.
111213
Our eyes are stimulated with various accommodative stimuli in everyday life, whether intended or not. The world is currently experiencing the Fourth Industrial Revolution (4IR). This is a next-generation industrial revolution in which advanced information and communication technologies such as artificial intelligence, Internet of Things (IoT), big data, and smart phones are being fused, resulting in a range of new technologies. The IoT, which refers to inter-networking of physical devices and computer-based systems, is representative of the 4IR, and smart glasses are one type of IoT. Several types of smart glasses such as Google Glasses (Google, Mountain View, CA, USA), Vuzix M300 (Vuzix, West Henrietta, NY, USA), and Optinvent ORA (Optinvent, Rue Patis Tatelin, France) have already been released and commercialized. These provide superimposed information on the field of view, such as global positioning systems, smart phone features, or activity trackers. Visual stimulation of only one eye through a single display and a prism could affect the contralateral eye. Therefore, the issue of how monocular visual stimulation causes any physiologic changes in the contralateral eye is important. Additionally, multiple-pinhole glasses have also been commercialized for the correction of near and distant vision in presbyopia. These glasses can be used irrespective of individual refraction status and without a specific prescription. However, no prior studies have investigated accommodative response to visual stimulation through pinhole glasses.
Accordingly, the purpose of the present study was to investigate physiological changes in accommodative power, pupil diameter, astigmatic value, and axis when visual stimuli were applied under binocular, monocular (dominant eye, non-dominant eye, ipsilateral, and contralateral), and pinhole conditions using the WAM-5500 binocular autorefractor/keratometer (Grand Seiko Co. Ltd., Hiroshima, Japan). We believe that this simple, clear, and objective study may contribute greatly to the understanding of accommodative responses during various accommodative stimulation conditions.
METHODS
Inclusion criteria were as follows: age between 20 and 40 years, spherical equivalent (SE) within ± 4.0 diopter (D), correctable distant and near visual acuity by glasses to 20/20, and normal ocular alignment. Exclusion criteria were as follows: disturbance of accommodation (e.g., Adie's pupil, Parkinson's disease, accommodative esotropia, accommodative spasm, and presbyopia), disorders that can affect accommodation (e.g., history of ocular trauma and previous ocular surgery), disorders that might limit testing accuracy (e.g., corneal pathological features, cataract, glaucoma, vitreous opacity, and retinal abnormalities), and any history of ocular surgery.
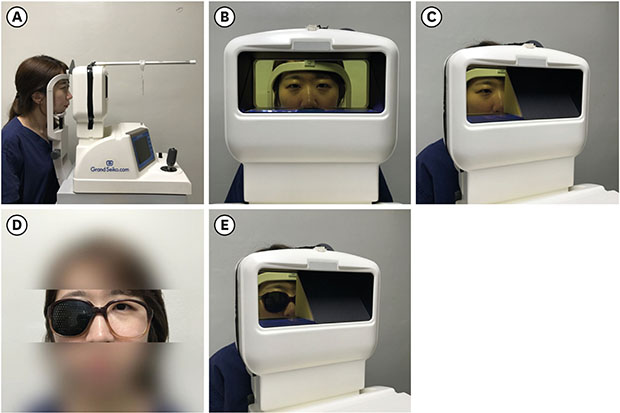
All subjects underwent basic ophthalmic examination including uncorrected distance visual acuity measurement, corrected distance visual acuity measurement, and an ocular dominance test. To evaluate normal ocular alignment, the patient was asked to follow a target to 9 diagnostic gaze positions while the physician checked for any abnormalities of the eye such as muscle weakness or overaction. Additionally, the cover-uncover test and alternative cover test were performed to check for manifest strabismus. The dominant eye was determined via the “hole in the card” test. Subjects were asked to hold a card with a 3 × 3 cm square hole with both hands. In the state of primary gaze, subjects were asked to observe a target at a distance of 4 m through the hole of the card. With one eye alternately covered, the eye through which the target was seen was determined as the dominant eye. In this study, a WAM-5500 binocular autorefractor/keratometer was used to evaluate objective accommodative power and pupil diameter modulation. The WAM-5500 provides a binocular open field of view which allows the subject to stare at the target through a transparent acrylate panel. We measured physiological changes while subjects, in a distance correction state, gazed at four refractive stimuli: 0.25 D (4 m distance), 2 D (50 cm), 3 D (33 cm), and 4 D (25 cm). The measurement was performed in 3 sets. One set was composed of 5 measurements, and the subjects were asked to focus on the center of the target to the best of their ability. They were offered a break, during which they were given plenty of time to rest and relax their eyes. Finally, the average value of a total of 15 measurements was recorded. All tests were conducted in the same room with 200 lux illumination and the target was used which corresponded to the international standard Snellen chart 20/20. There were three measurement conditions. First, we measured the response to binocular stimulation. While the subjects viewed the target in a binocular state, responses of each eye were measured. Secondly, we measured the response to monocular stimulation. The subjects viewed the target in a monocular state while the vision on the contralateral side was obscured with an opaque board. The responses of both the ipsilateral and contralateral eyes were measured, as it is possible to measure the physiologic changes in the obscured side through the inner window behind the opaque board. Thirdly, the response to pinhole glasses was measured. Pinhole glasses with 125 pinholes and with 0.9 mm-wide apertures were separated by 3 mm horizontally, 3 mm vertically, and 3.5 mm diagonally. To evaluate the accommodative response while wearing pinhole glasses, subjects wore the glasses on one side only and stared at the target with that eye, while the autorefractor measured the refraction state of the contralateral eye because measurements cannot be made with pinhole glasses on both eyes (
Fig. 1). We got the patient's permission to publish her photograph.
Fig. 1
Measurement of physiologic changes using the WAM-5500 binocular autorefractor/keratometer (Grand Seiko Co. Ltd., Hiroshima, Japan). (A) Measurement of physiologic changes under monocular and binocular stimuli. The target was located at four distances; 0.25 D (4 m distance), 2 D (50 cm), 3 D (33 cm), and 4 D (25 cm). (B) Open binocular field of view was provided through a transparent acrylic plate window. The autorefractor measured physiologic changes in the ipsilateral eye, with the contralateral eye covered by the opaque board, or physiologic changes in the contralateral eye through the inner window behind the opaque board. (C) The opaque board at the contralateral eye was used to prevent fixation on the near target. (D, E) Measurement of physiologic changes under pinhole stimulus. The refraction state could not be measured with the autorefractor while wearing pinhole glasses. Therefore, subjects wore the pinhole glasses on one side only and stared at the target with that eye, while the autorefractor measured the refraction state of the contralateral eye. We got the patient's permission to publish her photograph.
D = diopter.

The SE (sphere + 1/2 cylinder) was used to assess the response to refractive stimuli. Astigmatism was indicated with a minus sign. The principal meridian of the cylinder for 0.25 D refractive stimulus was set to the baseline axis. For 2 D, 3 D, and 4 D refractive stimuli, vertical movement was determined when the baseline meridian moved to the 90o axis, and it was considered horizontal movement when it moved to the 0o or 180o axis.
Statistical analyses were performed using SPSS software (version 19.0 for Windows; SPSS, Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant. Data from only the right eye of the subjects were used for analysis. Paired t-tests were used to compare differences in vision between monocular versus binocular, dominant eye versus non-dominant eye, ipsilateral versus contralateral, and monocular versus wearing pinhole glasses.
Ethics statement
This study was approved by the Chung-Ang University Hospital Institutional Review Board (IRB), Seoul, Korea (IRB No. C2016-2176) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants. This study was registered at
https://register.clinicaltrials.gov (Trial Registration No.
NCT03557346).
RESULTS
A total of 32 eyes of 32 subjects with a mean age of 27.22 (range, 24.72–29.72) years were included. Clinical and demographic characteristics of the subjects are presented in
Table 1. Of the 32 participants, 12 (37.5%) had myopia with SE over −0.5 D, 5 (15.6%) had hyperopia with SE over + 0.5 D, and 15 (46.9%) had emmetropia with SE within ± 0.5 D.
Table 1
Clinical and demographic characteristics of the subjects

|
Characteristics |
Value |
|
No. of subjects |
32 |
|
Gender, men:women |
14:18 |
|
Age, yr |
27.22 (24.72–29.72) |
|
SE, D |
−1.4 (−2.13, −0.67) |
|
Emetropia |
15 |
|
Hyperopia |
5 |
|
Myopia |
12 |
|
UDVA, logMAR |
0.16 (0.02–0.30) |
|
CDVA, logMAR |
0.00 (0–0) |
|
PinholeDVA, logMAR |
0.03 (−0.05, 0.11) |
|
Pupil diameter, mm |
2.87 (2.63–3.11) |
Response according to refractive stimuli under monocular and binocular conditions
When 2 D, 3 D, and 4 D stimuli were given, accommodative responses in the monocular stimuli were less than those in the binocular stimuli. Responses to monocular stimuli were 88% of the 2 D stimulus, 82% of the 3 D stimulus, and 83.8% of the 4 D stimulus, and the average response was 84.6% of the actual refractive stimulation. Responses to binocular stimuli were 92.5% of the 2 D stimulus, 89.3% of the 3 D stimulus, and 90.8% of the 4 D stimulus, and the average response was 90.9% of the actual refractive stimulation. In particular, 3 D stimulation (monocular = –2.46 D, binocular = –2.68 D;
P = 0.003) and 4 D stimulation (monocular = –3.35 D, binocular = –3.63 D;
P < 0.001) showed significant differences (
Fig. 2 and
Table 2).
Fig. 2
Physiologic changes by refractive stimuli in binocular and monocular stimuli. (A) Accommodative power under binocular and monocular according to refractive stimuli. (B) Pupil diameter under binocular and monocular according to refractive stimuli.
D = diopter.
aP value < 0.05 by paired t-test.

Table 2
Physiologic changes according to the refractive stimuli in monocular and binocular stimuli

|
Variables |
Monocular stimulus (n = 32) |
Binocular stimulus (n = 32) |
P valuea
|
|
Accommodative stimulation, D |
|
|
|
|
0.25 D |
−0.21 (−0.28, −0.14) |
−0.20 (−0.29, −0.10) |
0.401 |
|
2 D |
−1.76 (−1.88, −1.63) |
−1.85 (−1.95, −1.75) |
0.100 |
|
3 D |
−2.46 (−2.58, −2.39) |
−2.70 (−2.78, −2.59) |
0.013 |
|
4 D |
−3.35 (−3.47, −3.26) |
−3.63 (−3.73, −3.53) |
< 0.001 |
|
Pupil diameter, mm |
|
|
|
|
0.25 D |
3.12 (2.95–3.29) |
2.88 (2.72–3.03) |
< 0.001 |
|
2 D |
2.71 (2.57–2.91) |
2.40 (2.29–2.58) |
< 0.001 |
|
3 D |
2.34 (2.13–2.55) |
2.01 (1.80–2.17) |
< 0.001 |
|
4 D |
2.00 (1.82–2.18) |
1.62 (1.45–1.80) |
< 0.001 |
Pupil diameter decreased with increasing refractive stimulus in both monocular and binocular stimuli. A comparison of these stimuli showed that pupil diameter was larger in the monocular condition (3.12 mm, 2.71 mm, 2.34 mm, and 2.00 mm; all
P < 0.001) than in the binocular condition (2.88 mm, 2.40 mm, 2.01 mm, and 1.62 mm, respectively) (
Fig. 2 and
Table 2).
In summary, there was less accommodative response in both monocular and binocular stimuli than the actual refractive stimulus provided, and a larger accommodative response occurred in the binocular stimuli than the monocular stimuli.
Accommodative responses according to four monocular refractive stimuli
There were no significant differences in accommodative responses between dominant and non-dominant eye stimuli for 0.25 D, 2 D, 3 D, or 4 D monocular refractive stimuli. Similarly, there was no significant difference between ipsilateral and contralateral stimuli. That is, stimulation of one eye caused the same accommodative response in the other eye (
Table 3).
Table 3
Accommodative power according to refractive stimuli for four monocular stimuli conditions

|
Variables |
Dominant, eye stimulus (n = 32) |
Non-dominant, eye stimulus (n = 32) |
P valuea
|
Ipsilateral, eye stimulus (n = 32) |
Contralateral, eye stimulus (n = 32) |
P valueb
|
|
Accommodative stimulation, D |
|
|
|
|
|
|
|
0.25 D |
−0.22 (−0.37, −0.07) |
−0.27 (−0.40, −0.14) |
0.240 |
−0.24 (−0.38, −0.10) |
−0.14 (−0.22, −0.06) |
0.512 |
|
2 D |
−1.86 (−2.01, −1.71) |
−1.85 (−2.04, −1.66) |
0.887 |
−1.98 (−2.16, −1.80) |
−1.63 (−1.74, −1.52) |
0.897 |
|
3 D |
−2.46 (−2.62, −2.30) |
−2.44 (−2.62, −2.26) |
0.817 |
−2.59 (−2.77, −2.41) |
−2.56 (−2.69, −2.43) |
0.748 |
|
4 D |
−3.33 (−3.50, −3.16) |
−3.30 (−3.48, −3.12) |
0.500 |
−3.30 (−3.47, −3.13) |
−3.48 (−3.60, −3.36) |
0.872 |
Changes in astigmatic value and directionality in response to refractive stimuli
In the binocular stimulation state, the astigmatic value and axis in the far distance fixed state were set as baseline, and differences between baseline and values for the 2 D, 3 D, and 4 D stimuli were compared. Absolute astigmatism increased significantly with increasing refractive stimuli (0.17 D, 0.29 D, and 0.49 D for 2 D, 3 D, and 4 D stimuli, respectively; all
P < 0.001). Directional changes in the astigmatic axis in response to 2 D, 3 D, and 4 D refractive stimuli were evaluated as vertical or horizontal based on the astigmatic axis of the baseline. As the refractive stimulus increased, the horizontal direction increased. For 4 D stimulation, horizontal directional movement of the astigmatic axis was observed in 22 out of 32 eyes (68.8%) compared with the baseline response (
Table 4).
Table 4
Difference in astigmatic value and astigmatic axis between refractive stimuli and baseline (far)

|
Variables |
Astigmatic value, D |
P valuea
|
Direction of astigmatic axis |
|
Horizontal (%) |
Vertical (%) |
|
2 D-far |
−0.17 (−0.22, −0.13) |
< 0.001 |
15 (46.9) |
17 (53.1) |
|
3 D-far |
−0.29 (−0.33, −0.23) |
< 0.001 |
18 (56.3) |
14 (43.7) |
|
4 D-far |
−0.49 (−0.55, −0.37) |
< 0.001 |
22 (68.8) |
10 (31.2) |
Response according to refractive stimulus in monocular and pinhole glasses
Since the accommodative response was measured in the contralateral eye when pinhole glasses were worn, it was compared with the response obtained in the contralateral eye stimulus. For 2 D, 3 D, and 4 D stimuli, those wearing pinhole required less accommodative power (1.46 D, 2.20 D, and 2.96 D; all
P < 0.001) than the monocular (1.63 D, 2.56 D, and 3.48 D, respectively). That is, pseudo-accommodation by pinholes reduced the required accommodation by 10% to 15%. Pupil diameter decreased with increasing refractive stimuli in both groups. Pupil diameter was larger for the pinhole (4.11 mm, 3.74 mm, 3.41 mm, and 3.13 mm; all
P < 0.001) than the monocular (3.21 mm, 2.94 mm, 2.58 mm, and 2.23 mm, respectively) (
Table 5).
Table 5
Physiologic changes according to refractive stimuli under monocular and pinhole stimulus

|
Variables |
Monocular stimulus (n = 32) |
Pinhole stimulus (n = 32) |
P valuea
|
|
Accommodation, D |
|
|
|
|
Far |
−0.14 (−0.22, −0.06) |
−0.23 (−0.31, −0.15) |
0.005 |
|
2 D |
−1.63 (−1.74, −1.52) |
−1.46 (−1.56, −1.36) |
< 0.001 |
|
3 D |
−2.56 (−2.69, −2.43) |
−2.20 (−2.35, −2.05) |
< 0.001 |
|
4 D |
−3.48 (−3.60, −3.36) |
−2.96 (−3.07, −2.85) |
< 0.001 |
|
Pupil diameter, mm |
|
|
|
|
Far |
3.21 (3.01–3.41) |
4.11 (3.78–4.44) |
< 0.001 |
|
2 D |
2.94 (2.74–3.14) |
3.74 (3.41–4.07) |
< 0.001 |
|
3 D |
2.58 (2.34–2.82) |
3.41 (3.08–3.74) |
< 0.001 |
|
4 D |
2.23 (1.96–2.50) |
3.13 (2.80–3.46) |
< 0.001 |
DISCUSSION
In this study, we investigated the objective accommodative response and changes in pupil diameter in subjects with normal accommodation. Stimuli provided under the binocular condition induced more accommodative power and a smaller pupil diameter than those under the monocular condition. Furthermore, in the monocular condition, an equal accommodative response was induced in the contralateral eye. The absolute astigmatic value increased and direction changed horizontally as the near refractive stimulus became stronger. Pinhole glasses required less accommodative power compared with that in the absence of pinhole glasses.
When the eyes of subjects with normal accommodation received near refractive stimuli, the accommodative response was 90.9% of the refractive stimulus actually given. This 9.1% deficit in accommodative response is thought to be complemented by pseudo-accommodation, which involves changes in pupil diameter, changes in anterior chamber depth, and corneal aberrations.
1415 However, in the monocular stimulus, the accommodative response was 84.6% of the refractive stimulus actually provided, and the pupil diameter was also consistently larger than that in the binocular stimulus. That means that the 15.4% deficit in accommodative response induced by monocular stimuli may not be fully compensated for by pseudo-accommodation when compared with the binocular stimuli. Thus, in people with normal accommodation, more complete accommodation is achieved when stimulation is applied simultaneously to both eyes rather than to one eye. Binocular stimulation results in an effective accommodative response and efficient pupil response compared with monocular stimulation. The main reason for this is probably the convergence in which eyes rotate toward each other. This result must be considered when prescribing glasses, especially in cases of presbyopia.
We confirmed that there were no differences in response to refractive stimuli between the dominant eye and non-dominant eye and between the ipsilateral eye and contralateral eye. Even if the refractive stimulus is projected to one eye, it is transmitted to the Edinger-Westphal nucleus through the afferent limb, resulting in a similar occurrence of the accommodation reflex in both eyes. This is because the Edinger-Westphal nucleus sends axons to the contralateral ocular motor and ciliary ganglion as well.
10 This study revealed that ocular dominance is determined by the tendency to prefer visual input or the eye's refractive status, not by the accommodation ability. Therefore, smart glasses should not be used while driving or in cases where accommodative function is decreased (e.g., presbyopia), because monocular stimulation provided by smart glasses would induce the same degree of accommodative response in the contralateral eye. Problems related to cognitive distraction due to images induced by stimulating one eye and the unintentional accommodative response in the contralateral eye must be considered when developing regulations for the use of smart glasses. An attempt has been made to equip some smart glasses with the ability to minimize the accommodation in the contralateral eye by incorporating a plus lens into the optical pathway. However, the use of smart glasses definitely affects the movement of the muscles of the contralateral eye, considering that the accommodative function of each person is different. Therefore, there is a limit to minimizing the accommodation of the contralateral eye by coupling with a plus lens only. Additionally, since it is known that the dominant eye exerts greater accommodative response when aniso-accommodative stimuli is provided in binocular viewing,
13 the position of the prism and plus lens in smart glasses must be considered.
Astigmatism is based on genetic factors and is influenced by the interaction of extraocular muscles, visual feedback, and eyelid pressures.
161718 Several studies have found that convergence causes changes in corneal topography.
1920 Visual tasks involving downgazing during reading lead to changes in the position, angle, and tension of lids. More horizontal eye movements, narrower palpebral apertures, and more increased lid tension exhibit more localized corneal changes consistent with against-the-rule astigmatism. Therefore, we should consider the effect of lid changes. However, we measured the refractive changes not by downgaze, but by looking straight ahead through the autorefractor. We assume that effects of eyelids on change in astigmatism were minimal in this study.
212223 When convergence occurs, the tension in the medial rectus muscle increases, which makes the horizontal portion of the cornea flatter. In this study, we confirmed that the absolute value of astigmatism increased as the near refractive stimulus became stronger, and the number of cases in which the astigmatism axis moved horizontally increased. These changes in astigmatism serve as a kind of pseudo-accommodation. This also implies that the primary function of convergence is to change the visual axis to focus an object located at a near distance, but also contributes to accommodation.
The required accommodative power over the given refractive stimulus was statistically lower for pinhole stimuli than monocular stimuli. Pinhole stimuli required 10.4% less accommodative power for 2 D, 14% less accommodative power for 3 D, and 14.9% less accommodative power for 4 D compared to monocular stimuli. However, due to the opaque glass plate of pinhole glasses, luminance decreased and the pupil diameter behind pinhole glasses increased. The fact that the diameter of the pupil increased implies that pseudo-accommodation by the pupil decreased. Nevertheless, wearing pinhole glasses required less accommodative power.
2425 This is because pseudo-accommodation in response to the pinhole was large enough to negate the reduction of pseudo-accommodation by the pupil. The multiple small apertures of pinhole glasses reduce the blur circle, which is a type of pseudo-accommodation,
26 resulting in 10%–15% reduction in the required accommodative power compared to monocular stimuli. However, it is worth considering that the limitation that an autorefractor/keratometer sometimes produces are inaccurate.
This prospective, non-randomized clinical trial investigated physiologic changes during accommodation objectively. Binocular stimuli enable more precise and effective accommodation than do monocular stimuli. At each distance, true accommodation was about 90% while pseudo-accommodation may account for the remaining 10%. As the refractive stimuli became stronger, the absolute astigmatic value increased and the direction of the astigmatism axis became more horizontal. If near stimulation is applied to only one eye when wearing smart glasses, there will be approximately 85% accommodation of the actual stimulus in the contralateral eye. True and pseudo-accommodative responses, change of the astigmatism value, and axis direction to refractive stimuli are factors that should be considered when developing guidelines for wearing smart glasses.











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download