1. Tseng WT, Yen CT, Tsai ML. A bundled microwire array for long-term chronic single-unit recording in deep brain regions of behaving rats. J Neurosci Methods. 2011; 201(2):368–376.


2. Wark HA, Sharma R, Mathews KS, Fernandez E, Yoo J, Christensen B, et al. A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J Neural Eng. 2013; 10(4):045003.
3. Kee-Keun L, Jiping H, Amarjit S, Stephen M, Gholamreza E, Bruce K, et al. Polyimide-based intracortical neural implant with improved structural stiffness. J Micromech Microeng. 2004; 14(1):32–37.
4. Durand DM, Ghovanloo M, Krames E. Time to address the problems at the neural interface. J Neural Eng. 2014; 11(2):020201.

6. Kozai TD, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, et al. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J Neural Eng. 2010; 7(4):046011.
7. Johnson MD, Kao OE, Kipke DR. Spatiotemporal pH dynamics following insertion of neural microelectrode arrays. J Neurosci Methods. 2007; 160(2):276–287.


8. Thelin J, Jörntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N, et al. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS One. 2011; 6(1):e16267.

9. Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, et al. Brain responses to micro-machined silicon devices. Brain Res. 2003; 983(1-2):23–35.


10. Thelin J, Jörntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N, et al. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS One. 2011; 6(1):e16267.

11. Eriksson Linsmeier C, Prinz CN, Pettersson LM, Caroff P, Samuelson L, Schouenborg J, et al. Nanowire biocompatibility in the brain--looking for a needle in a 3D stack. Nano Lett. 2009; 9(12):4184–4190.

13. Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007; 28(25):3594–3607.


14. Stice P, Gilletti A, Panitch A, Muthuswamy J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J Neural Eng. 2007; 4(2):42–53.


15. Kozai TD, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012; 11(12):1065–1073.

16. Patel PR, Na K, Zhang H, Kozai TD, Kotov NA, Yoon E, et al. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J Neural Eng. 2015; 12(4):046009.
17. Patel PR, Zhang H, Robbins MT, Nofar JB, Marshall SP, Kobylarek MJ, et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J Neural Eng. 2016; 13(6):066002.
18. Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. A carbon-fiber electrode array for long-term neural recording. J Neural Eng. 2013; 10(4):046016.

19. Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng. 2004; 51(6):896–904.

20. Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006; 3(1):59–70.


21. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003; 24(3):401–416.


22. Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005; 26(2):147–155.

24. Ludwig KA, Miriani RM, Langhals NB, Joseph MD, Anderson DJ, Kipke DR. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. J Neurophysiol. 2009; 101(3):1679–1689.


25. Strand AM, Venton BJ. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Anal Chem. 2008; 80(10):3708–3715.


26. Prasad A, Sanchez JC. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng. 2012; 9(2):026028.
27. Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005; 148(1):1–18.


28. Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A. 2007; 82(1):169–178.


29. Liu X, McCreery DB, Carter RR, Bullara LA, Yuen TG, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999; 7(3):315–326.

30. Roitbak T, Syková E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999; 28(1):40–48.


31. Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, et al. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999; 156(1):33–49.


33. Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng. 1992; 39(6):635–643.


34. Lin YC, Ramadan M, Hronik-Tupaj M, Kaplan DL, Philips BJ, Sivak W, et al. Spatially controlled delivery of neurotrophic factors in silk fibroin-based nerve conduits for peripheral nerve repair. Ann Plast Surg. 2011; 67(2):147–155.


36. Wittmer CR, Claudepierre T, Reber M, Wiedemann P, Garlick JA, Kaplan D, et al. Multifunctionalized electrospun silk fibers promote axon regeneration in central nervous system. Adv Funct Mater. 2011; 21(22):4202.


37. Abbott RD, Kimmerling EP, Cairns DM, Kaplan DL. Silk as a biomaterial to support long-term three-dimensional tissue cultures. ACS Appl Mater Interfaces. 2016; 8(34):21861–21868.


38. Wu F, Tien LW, Chen F, Berke JD, Kaplan DL, Yoon E. Silk-backed structural optimization of high-density flexible intracortical neural probes. J Microelectromech Syst. 2015; 24(1):62–69.

39. Liu B, Song YW, Jin L, Wang ZJ, Pu DY, Lin SQ, et al. Silk structure and degradation. Colloids Surf B Biointerfaces. 2015; 131:122–128.



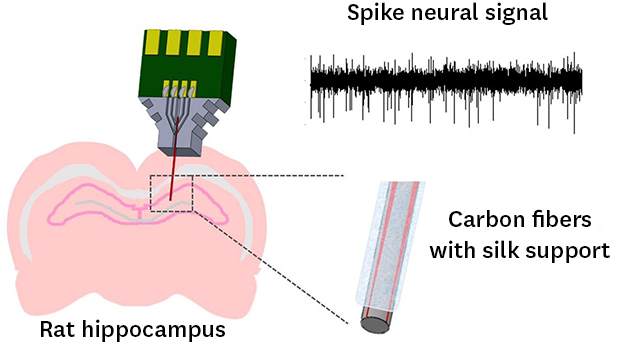
 , x = band-pass filtered signal). And then with a sampling frequency of 25 kHz, this plots 64 datapoints. Spike waveforms were overlapped and the mean values at each time point are extracted as sorted signal.
, x = band-pass filtered signal). And then with a sampling frequency of 25 kHz, this plots 64 datapoints. Spike waveforms were overlapped and the mean values at each time point are extracted as sorted signal.






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download