Abstract
Betula platyphylla var. japonica (Betulaceae), also known as Asian white birch, is an endemic medicinal tree, the bark of which has been used in Chinese traditional medicine for the treatment of various inflammatory diseases. In our continuing search for bioactive compounds from Korean natural resources, a phytochemical investigation of the bark of B. platyphylla var. japonica led to the isolation of 7-oxo-β-sitosterol (1) and soyacerebroside I (2) from its ethanol extract as main components by liquid chromatography (LC)/mass spectrometry (MS)-based analysis. The structures of isolates were identified by comparison of 1H and 13C nuclear magnetic resonance spectroscopic data and physical data with the previously reported values and LC/MS analyses. To the best of our knowledge, this is the first study to demonstrate that the isolated compounds, 7-oxo-β-sitosterol and soyacerebroside I, were isolated in B. platyphylla var. japonica. We examined the effects of the isolates on the regulation of adipocytes and osteoblast differentiation. These isolates (1 and 2) produced fewer lipid droplets compared to the untreated negative control in Oil Red O staining of the mouse mesenchymal stem cell line without altering the amount of alkaline phosphatase staining. The results demonstrated that both compounds showed marginal inhibitory effects on adipocyte differentiation but did not affect osteoblast differentiation.
Betula platyphylla var. japonica (Miquel) Hara (Betulaceae), commonly called Asian white birch, is widely distributed across Korea, Japan, China, and eastern Siberia. Its bark has been used by native Asians as a traditional medicine for various inflammatory diseases such as pneumonia, choloplania, nephritis, and chronic bronchitis.1 A recent study reported that dammarane-type triterpenoids derived from the extract of the bark of B. platyphylla var. japonica displayed cytotoxic activity against multidrug-resistant cancer cell lines (KB-C2 or K562/Adr).2 Also, another study reported that platyphylloside, a diarylheptanoid isolated from B. platyphylla, decreased adipocyte differentiation and induced lipolysis in 3T3-L1 cells.3
As part of a continuing search for bioactive constituents from Korean medicinal plant sources, we have taken an interest in bioactive compounds from the bark of B. platyphylla var. japonica.45 In our recent studies, we reported the isolation of antioxidant triterpenoids combined with phenylpropanoid units and cytotoxic triterpenoids against several human tumor cells.45 In this study, we further investigated possible new pharmacological effects of components from the bark of B. platyphylla var. japonica, and two chemical components (1 - 2) were isolated from the ethanol (EtOH) extract of the bark through liquid chromatography (LC)/mass spectrometry (MS)-based analysis. We examined the regulatory effects of isolates (1 - 2) on the differentiation of mesenchymal stem cells (MSCs) into adipocytes and osteoblasts. We describe here the details of the LC/MS-guided isolation and identification of compounds (1 - 2) as well as their effects on the regulation of adipocyte and osteoblast differentiation.
Optical rotations were measured on a Jasco P-1020 polarimeter (Jasco, Easton, MD, USA). Infrared (IR) spectra were recorded on a Bruker IFS-66/S FT-IR spectrometer (Bruker, Karlsruhe, Germany). Ultraviolet (UV) spectra were acquired on an Agilent 8453 UV-visible (UV-Vis) spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE III with chemical shifts given in ppm (δ) (Bruker). Semi-preparative high-performance liquid chromatography (HPLC) was carried out using a Shimadzu Prominence HPLC System with SPD-20A/20AV Series Prominence HPLC UV-Vis Detectors (Shimadzu, Tokyo, Japan). LC/MS analysis was performed on an Agilent 1200 Series HPLC system equipped with a diode array detector and a 6130 Series electrospray ionization mass spectrometer (ESI-MS) using an analytical Kinetex® 5-µm C18 100 Å column (5 µm, 2.1 × 100 mm, Phenomenex, Torrance, CA, USA). Column chromatography was performed with Silica gel 60 (Merck, Darmstadt, Germany; 230 – 400 mesh) and RP-C18 silica gel (Merck, 230 – 400 mesh). Merck precoated silica gel F254 plates and reverse-phase (RP)-18 F254s plates (Merck) were used for thin-layer chromatography (TLC). Spots were detected on TLC under UV light or by heating after spraying with anisaldehyde–sulfuric acid.
The bark of B. platyphylla var. japonica was collected from Danyang, Chungcheongbuk-do, Korea, in October 2014. The material was identified by one of the authors (K. H. Kim). A voucher specimen (NM-14-063) was deposited in the herbarium of the Natural Medicine Research Center of Richwood Pharmaceutical Company, Ltd., Seoul, Korea.
Dried bark of B. platyphylla var. japonica (4.1 kg) was extracted with 80% EtOH (18 L × 1 day × three times) at room temperature and filtered. The resultant solution was evaporated under reduced pressure using a rotavapor to obtain the EtOH extract (351 g), which was suspended in distilled water (2 L) and successively solvent-partitioned with CHCl3, ethyl acetate, and n-butanol (BuOH), yielding residues weighing 274 g, 25 g, and 30 g, respectively.
According to the LC/MS analysis of each fraction, two peaks with molecular ion peaks at m/z 429 [M+H]+ and 714 [M+H]+ were detected in the n-BuOH-soluble fraction, and they turned out to be compounds that have not been reported from B. platyphylla var. japonica. The n-BuOH-soluble fraction (30 g) was applied to silica gel open column chromatography using a gradient solvent system of CH2Cl2/methanol (MeOH) (10:1), CH2Cl2/MeOH/H2O (7:4:1), and 100% MeOH to give three fractions (Frs. B1 – B3). The target peaks were isolated by open-column chromatography and semi-preparative HPLC monitored by LC/MS analysis. The peak with the molecular ion peak at m/z 429 [M+H]+ was present in Fr. B1 (4.6 g), which was separated by silica gel open column chromatography with a gradient solvent system of CH2Cl2/MeOH/H2O (7:3:0.5, 7:4:1) to obtain five fractions (Frs. B1a – B1e). Fr. B1a (78.7 mg), containing the peak, was purified by semi-preparative RP HPLC with a solvent system of MeOH/H2O (97:3) using a Phenomenex Luna C18(2) column (250 mm × 10 mm i.d., 10 µm) to yield compound 1 (2.5 mg).
Another peak with the molecular ion peak at m/z 714 [M+H]+ was present in Fr. B2 (7.9 g), which was separated by silica gel open column chromatography using a gradient solvent system of CH2Cl2/MeOH/H2O (7:3:0.1, 7:4:1) to obtain six fractions (Frs. B2a – B2f). Fr. B2a (337.5 mg), containing the peak, was separated by preparative RP HPLC with a gradient solvent system of MeOH/H2O (65:35, 1:0) using an Agilent Eclipse XDB-C18 column (250 mm × 21.2 mm i.d., 7 µm) to yield seven fractions (Frs. B2a-1 – B2a-7). Fr. B2a-2 (14.0 mg) containing the peak was purified by semi-preparative RP HPLC with a solvent system of MeOH/H2O (6:4) using a Phenomenex Luna C18(2) column (250 mm × 10 mm i.d., 10 µm) to afford compound 2 (4.2 mg).
White powder, ESI-MS m/z 429 [M+H]+; 1H NMR (CDCl3, 600 MHz): δ 0.66 (3H, s, H-18), 0.81 (3H, t, J = 6.8 Hz, H-29), 0.85 (3H, d, J = 6.8 Hz, H-27), 0.93 (3H, d, J = 6.5 Hz, H-21), 1.19 (3H, s, H-19), 2.03 (1H, dt, J = 12.7, 4.0 Hz, H-12β), 2.23 (1H, t, J = 11.8 Hz, H-8), 2.40 (1H, ddt, J = 13.0, 11.0, 1.8 Hz, H-4β), 2.50 (1H, ddd, J = 13.0, 5.0, 1.8 Hz, H-4α), 3.67 (1H, tt, J = 11.0, 5.0 Hz, H-3), 5.68 (1H, br s, H-6); 13C NMR (CDCl3, 150 MHz): δ 202.3 (C-7), 165.0 (C-5), 126. 1 (C-6), 70.0 (C-3), 54.7 (C-17), 49.9 (C-9, 14), 45.8 (C-24), 45.4 (C-8), 43.1 (C- 13), 41.8 (C-4), 38.7 (C-12), 38.3 (C-10), 36.4 (C-1), 36.l (C-20), 33.9 (C-22), 31.2 (C-2), 29.1 (C-25), 28.5 (C-16), 26.3 (C-15), 26.1 (C-23), 23.0 (C-28), 21.2 (C-11), 19.8 (C-27), 19.0 (C-26), 18.9 (C-21), 17.3 (C-19), 11.9 (C-18, 29).
White amorphous powder; ESI-MS m/z 714 [M+H]+; 1H NMR (pyridine-d5, 500 MHz): δ 0.86 (6H, t-like, J = 6.9 Hz, H-18 and H-16′), 1.25–1.35 (38H, m, 19 × CH2), 1.25–1.35 (38H, m, 19 × CH2), 1.37 (1H, m, H-4′), 1.71 (1H, m, H-3′), 1.99 (2H, m, H-10), 2.14 (4H, br s, H-6 and H-7), 3.92 (2H, m, H-2′ and H-5″), 4.05 (4H, m, H-1b, H-2, H-3 and H-2″), 4.21 (1H, m, H-4″), 4.25 (2H, m, H-1a and H-3″), 4.38 (1H, m, H-6″a), 4.51 (1H, m, H-6″b) , 4.92 (1H, d, J = 7.7 Hz, H-1″), 5.49 (3H, m, H-4, H-8 and H-9), 5.77 (1H, m, H-5), 8.38 (1H, d, J = 8.8 Hz, NH); 13CNMR (pyridine-d5, 125 MHz): δ 175.7 (C-1′), 132.1 (C-5), 132.1 (C-9), 131.1 (C-4), 130.0 (C-8), 105.7 (C-1), 78.6 (C-5), 78.5 (C-3), 75.2 (C-2), 72.3 (C-2′), 71.5 (C-3), 71.5 (C-4), 70.2 (C-1), 62.7 (C-6), 54.6 (C-2), 35.7 (C-3′), 32.9 (C-10), 32.2 (C-6), 32.1 (C-7), 29.6-30.0 (C-11-16 and C-5′-14′), 25.9 (C-4′), 23.0 (C-17 and C-15′), 14.3 (C-18 and C-16′).
The C3H10T1/2 cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and antibiotics (penicillin and streptomycin, Hyclone) and were incubated at 37 ℃ under 5% CO2 as described previously.6 For adipocyte differentiation, C3H10T1/2 cells were seeded at 2.5 × 104/mL in 6-well tissue culture plates, and then confluent cells were incubated for two days in DMEM supplemented with 10% FBS, 1 µM dexamethasone (Sigma, St. Louis, MO, USA), 0.5 mM isobutyl-1-methylxanthine (Sigma), 10 µM troglitazone (Sigma), and 5 µg/mL insulin (Sigma). Cells were refreshed with DMEM containing 10% FBS, 10 µM and 5 µg/mL insulin every 3 days for 8 days. Osteoblast differentiation media, which consisted of DMEM, 5% FBS, 50 µg/mL ascorbic acid (Sigma), and 10 mM β-glycerophosphate (Sigma) was changed every 3 days for 9 days.
At 6 – 8 days after differentiation, cells were fixed with 10% neutral buffered formalin (NBF) for 60 min, then stained with 0.5% ORO (Sigma) in a mixture of isopropanol and distilled water at a 3:2 ratio for 60 min. Cells were washed three times with water, then photographed under a microscope or scanned. To quantify intracellular triglyceride content, stained cells from at least two independent experiments were resolved with 1 mL isopropanol and measured with a spectrophotometer at 520 nm.
After osteogenic differentiation for 7 – 9 days, the medium was removed, and the cells were rinsed with 2 mM/L MgCl2. The cells were incubated with alkaline phosphatase buffer (100 mM/L Tris-HCl pH 9.5, 100 mM/L NaCl, and 10 mM/L MgCl2) for 15 min. They were then incubated in alkaline phosphatase buffer containing 0.4 mg/mL nitro-blue tetrazolium (Sigma) and 0.2 mg/mL 5-bromo-4-chloro-3-indolyl phosphate (Sigma). The reaction was stopped by adding 5 mM/L ethylenediaminetetraacetic acid (pH 8.0). The cells were fixed in 10% NBF for 60 min and then rinsed three times with distilled water.
Betula platyphylla var. japonica bark was extracted with 80% EtOH and then filtered. After evaporation of the filtrate, the resultant EtOH extract was obtained. We then fractionated the EtOH extract into three different fractions, the CHCl3, ethyl acetate, and n-BuOH-soluble fractions. By comparison with our house-built UV library, LC/MS analysis of the three fractions indicated the presence of two peaks with molecular ion peaks at m/z 429 [M+H]+ and 714 [M+H]+ in the n-BuOH-soluble fraction (Fig. 1), and they turned out to be compounds that have not been previously reported from B. platyphylla var. japonica. The high sensitivity and selectivity of the LC/MS-guided isolation method selectively reduced the analysis time and enabled the fast isolation of target compounds. The target compounds in the n-BuOH-soluble fraction were isolated by open-column chromatography and semi-preparative HPLC monitored by LC/MS analysis, which led to the successful isolation of the target compounds. The isolated compounds were identified as 7-oxo-β-sitosterol (1)7 and soyacerebroside I (2)8 by spectroscopic methods, including 1H and 13C NMR and LC/MS analysis, and comparison of the spectroscopic and physical data with previously reported results (Fig. 1). To the best of our knowledge, this is the first study to demonstrate that the isolated compounds, 7-oxo-β-sitosterol and soyacerebroside I, have been isolated from B. platyphylla var. japonica.
To identify the regulatory effects of the isolates (1 and 2) on differentiation into adipocytes and osteoblasts, various concentrations of the isolated compounds were treated in cultured mouse MSCs of the C3H10T1/2 cell line during adipogenesis or osteogenesis. MDI (3-isobutyl-1-methylxanthine, dexamethasone, and insulin)-induced differentiation into adipocytes of MSC was slightly inhibited by 10 µM of the isolates. These compounds inhibited lipid formation in the MSCs at levels comparable to the positive control, 20 µM resveratrol (Fig. 2). This study suggests that the isolated compounds, 7-oxo-β-sitosterol and soyacerebroside I, may be able to inhibit the accumulation of fat in the body.
To investigate the effects of compounds on the early stage of osteoblast differentiation, each compound was added to the MSC culture medium during osteogenesis for 10 days, and cells were stained for ALP expression (Fig. 2). As a result, the intensity of ALP staining from the compound-treated cells did not differ from the intensity in the untreated negative control. These results demonstrated that neither compound affected ALP activity or osteogenesis in the MSC differentiation.
Since the isolated compounds, 7-oxo-β-sitosterol and soyacerebroside I, demonstrated marginal anti-adipogenic effects, various concentrations of the compounds were tested in lipid droplet production (Fig. 3). After the differentiation, the degree of differentiation and lipid droplet production according to the concentration of the compounds was evaluated by ORO staining and was quantified by resolving in iso-propanol. However, there were no differences in the production of lipid droplets as concentrations were varied in either compound. Regardless of the concentrations of the two compounds 1 and 2, the treated cells showed 40 – 60% inhibition of adipocyte differentiation compared to the negative control. Also, at all concentrations of both compounds, the regulatory effect of MSC differentiation toward adipocytes did not reach that of the positive control, resveratrol (20 µM).
Although neither of the two compounds identified from B. platyphylla var. japonica was superior to the positive control, resveratrol, in terms of suppressive effects on adipocyte differentiation, it can be expected that the activity from the combined use of the two compounds would be higher than that of each of them individually. Likewise, it is possible to expect some anti-obesity activity of B. platyphylla var. japonica extract through inhibition of adipocyte differentiation, since the extract contains various components including the isolated compounds (7-oxo-β-sitosterol and soyacerebroside I) proven able to inhibit adipocyte differentiation.
Figures and Tables
 | Fig. 1LC/MS-based analysis of the EtOH extract and n-BuOH-soluble fraction as well as the chemical structures of compounds 1 and 2. (a) TIC chromatogram of the EtOH extract in the positive ESI-MS mode; (b) TIC chromatogram of n-BuOH-soluble fraction in the positive ESI-MS mode; (c) MS data and UV pattern of compound 1 in LC/MS analysis; (d) MS data and UV pattern of compound 2 in LC/MS analysis; (e) the chemical structures of compounds 1 and 2. |
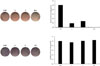 | Fig. 2Reciprocal effects of compounds 1 and 2 on the differentiation of MSCs toward adipocytes or osteoblasts. C3H10T1/2 cells, a mouse mesenchymal stem cell line, were treated with the compounds isolated from B. platyphylla var. japonica. After adipogenic or osteogenic differentiation, the cells were stained with ORO for adipocytes or with ALP for osteoblasts. Ctrl represents the untreated negative control. The positive control in adipogenesis was 20 µM of resveratrol (Res), and 10 µM of 17β-estradiol (E2) was added in the experimental set as a positive control in osteogenesis. In the treatment groups, 10 µM of each compound was added to the adipogenic or osteogenic differentiation medium. |
 | Fig. 3Suppressive effects of compounds 1 and 2 on adipogenic differentiation. Sequential concentrations of compounds 1 and 2 were administered to the C3H10T1/2 cells in culture media for nine days prior to ORO staining. The positive control, marked, Res, was 20 µM of resveratrol. The values were scaled by setting the untreated negative control to 1 and the positive control of 20 µM of resveratrol to 0. |
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A02037383) and by the Ministry of Education (NRF-2012R1A5A2A28671860).
References
1. Jiangsu New Medical College. Chinese Material Medica. Shanghai: Shanghai People's Publishing House;1977. p. 1784.
2. Kashiwada Y, Sekiya M, Yamazaki K, Ikeshiro Y, Fujioka T, Yamagishi T, Kitagawa S, Takaishi Y. J Nat Prod. 2007; 70:623–627.
3. Lee M, Sung SH.
. Pharmacogn Mag. 2016; 12:276–281.
4. Eom HJ, Kang HR, Choi SU, Kim KH. Chem Biodivers. 2017; 14:e1600400.
5. Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH. Bioorg Chem. 2016; 66:97–101.
6. Kang HR, Yun HS, Lee TK, Lee S, Kim SH, Moon E, Park KM, Kim KH. J Agric Food Chem. 2018; 66:2677–2684.
7. Ma XL, Lin WB, Zhang GL. Nat Prod Res Dev. 2009; 21:593–599.
8. Tang J, Meng X, Liu H, Zhao J, Zhou L, Qiu M, Zhang X, Yu Z, Yang F. Molecules. 2010; 15:9288–9297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download