INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory disease with a long disease duration. RA patients undergo long-term disease-modifying anti-rheumatic drugs (DMARDs) therapy and may suffer from various comorbidities, such as malignancy, cardiovascular disease, and lung disease. Several epidemiologic studies have suggested an association between RA and malignancy.
1234 Nevertheless, it remains unclear whether the overall cancer incidence rate is increased in RA patients: several studies have shown an increased overall cancer risk in RA patients, although some studies have reported no increase in overall cancer incidence.
567
Regarding site-specific cancers, several studies have reported that the incidence of lung cancer and lymphoma is greater in RA patients.
248 Simon, et al.
4 performed a meta-analysis of the incidence of malignancy in adult patients with RA and found that RA patients had increased risks of lymphoma and lung cancer and decreased risks of colorectal and breast cancers. There are no consistent findings on the risk of cervical cancer, prostate cancer, and melanoma.
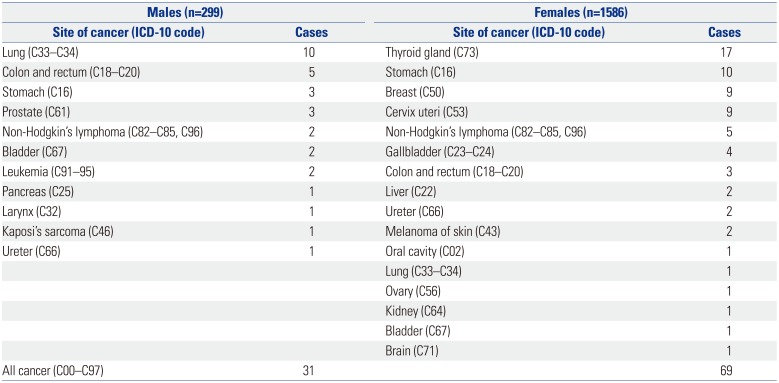
The incidences of specific malignancies are affected by a patient's region, race, age, sex, and times. In men, the five leading primary sites of malignancy were the stomach, lung, liver, colon and rectum, and bladder in 1999–2001.
9 Since then, the number of cases of colon cancer has increased, with colon cancer becoming the third most common cancer in 2015.
10 In women, the five leading primary sites of malignancy were the stomach, breast, colon and rectum, uterine cervix, and lung in 1999–2001.
9 Since then, the numbers of cases of thyroid cancer have increased rapidly, and thyroid cancer became the most common cancer in 2015.
10 In Korea, studies have been conducted on the incidence of malignancies in RA patients using biologic DMARDs; however, there has been no study on the risk of malignancy in RA patients versus the general population.
1112
In this study, we investigated the overall cancer risk, as well as the risks for specific cancer types, in RA patients in Korea by comparing the incidence of cancer among RA patients versus that in the general population.
Go to :

DISCUSSION
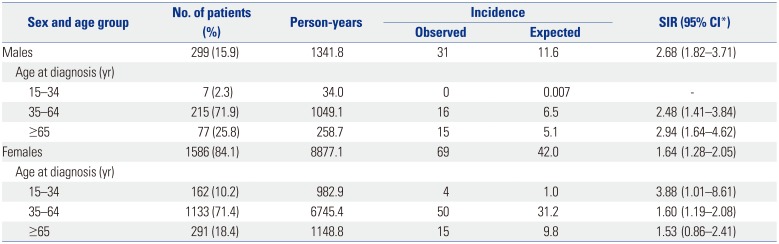
Medical records at a single tertiary hospital from 1996 through 2009 were reviewed, and 100 patients were diagnosed with cancer from 1885 RA patients. We compared the incidence of cancer according to sex because cancer incidence in the general population differs according to sex.
91013 In this study, male and female RA patients had increased risks of malignancy, and notably male patients showed a greater risk (SIR 2.68, 95% CI: 1.82–3.71) than female patients (SIR 1.64, 95% CI: 1.28–2.05). Male RA patients aged ≥65 years showed the highest incidence rate of cancer, while the incidence of cancer was highest in female RA patients aged ≤34 years. Chen, et al.
3 showed a slightly increased overall cancer risk in patients with RA (SIR 1.23, 95% CI: 1.22–1.23), and the risk was similar between women and men in Taiwanese patients with RA. On the other hand, Parikh-Patel, et al.
1 reported that the overall cancer risk in men was similar to that observed in the general population. The overall cancer risk in women was 15% lower than that of the general California population. Although individual studies have not shown consistent results, Simon, et al.
4 recently performed a meta-analysis and revealed that patients with RA have a 10% increase in overall malignancy risk compared with the general population.
The increased risk of hematological malignancies in RA patients is well known, and notably several studies have reported an increase in risk of lymphoma in patients with RA.
8 In our study, seven lymphoid malignancies (2 men and 5 women) were identified during the follow-up period. The SIR of lymphoid malignancies was increased in female RA patients (SIR 6.47, 95% CI: 2.04–13.4), although the difference was not significant in men (SIR 9.36, 95% CI: 0.88–26.8). During the follow-up period, two leukemia cases were identified in male RA patients, although no myeloid malignancy was observed in female RA patients. The SIR of leukemia was increased in male RA patients (SIR 16.7, 95% CI: 1.58–47.9). Similar results have been reported in previous studies. Lin, et al.
15 reported a significantly higher incidence of both lymphoid and myeloid malignancies in male RA patients compared with that in RA-free patients in Taiwan. In female RA patients, a significantly increased overall incidence risk was noted in lymphoid malignancies, although no significant increase was noted in myeloid malignancies. The incidence of malignant lymphoma was significantly increased in both male and female RA patients compared to that in the general population in a Japanese study.
2 Several possible reasons have been attributed to the increased risk of malignant lymphomas in RA patients. For instance, underlying inflammation, the existence of shared genetic risk factors, and an immunosuppressive treatment have been suggested as risk factors for RA-associated lymphomas. Recent studies have indicated an association between disease severity and lymphoma risk in RA.
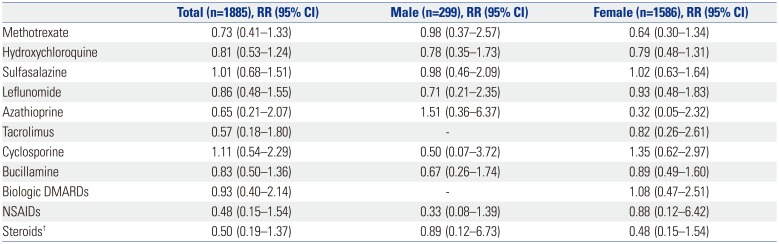
151617 Only a few studies have shown that RA patients have an increased risk of myeloid malignancies. Some cases have reported that myeloid malignancies could be caused by immune-modulating treatments, such as azathioprine or methotrexate, in autoimmune diseases.
1819
In our study, lung cancer occurred in 10 of 299 male RA patients and in one out of 1586 female patients. The risk of lung cancer was elevated in male RA patients (SIR 5.46, 95% CI: 2.60–9.36). The incidence of lung cancer in RA patients is known to be elevated in comparison to that in the general population. Simon, et al.
4 reported that RA patients have an increased risk of lung cancer compared with the general population. The SIRs for lung cancer ranged from 1.36 to 2.9. A retrospective cohort study conducted in RA patients in California revealed lung cancer risk to be elevated in both male (SIR=1.65, 95% CI:1.49–1.81) and female (SIR=1.28, 95% CI: 1.19–1.38) RA patients.
1 While no data were obtained on smoking in this study, other studies have reported smoking rates among the general population in the United States. In 2011, an estimated 19% of adults were current smokers, with a smoking prevalence of 21.6% among men and 16.5% among women.
20 In our study, the incidence of lung cancer was significantly higher in male RA patients. This result seems to be related to the high smoking rate among men in Korea. According to the report of the Ministry of Health and Welfare in Korea, 27.2% of adults aged >19 years were smokers, and the smoking rate of Korean men was 46.9% in 2009. In the study of the Korean Longitudinal Study on Health and Aging (KLo-SHA), the prevalences of ever-smoker and current-smoker women were 8.5% (95% CI: 5.8–11.2) and 3.9% (95% CI: 2.0–5.7), respectively.
21 In our study, eight of 11 lung cancer patients were smokers. Of the 11 patients, seven reported emphysema, and four had interstitial lung disease. In a South European population study, the 7 patients who developed lung cancer were men, smokers, and aged >55 years with a longstanding disease. The authors proposed that variables of being male, increasing age, previous use of cytotoxic, excluding methotrexate, erythrocyte sedimentation rate, and white blood cell count, were associated with a higher risk of developing cancer.
2223
The incidence of thyroid cancer was increased in female RA patients compared to that in the general population. In previous studies, the incidence of thyroid cancer was not significantly different from that in the general population.
123 It is difficult to generalize whether the incidence of thyroid cancer is higher in female RA patients using only the results of this study. In Korea, the incidence of thyroid cancer has rapidly increased in the general population, with increasing numbers of people undergoing thyroid cancer screening by ultrasonography. In 2014, the overdiagnosis of thyroid cancer was an issue.
24 RA patients frequently visit hospitals, which increases the chances of receiving thyroid cancer screening, which could have served as a bias in the study.
In this study, the risk of cervical cancer was greater in RA patients than in the general population (SIR=3.65, 95% CI: 1.65–6.42). Previous studies in Taiwan, California, and Japan did not show an increased incidence of cervical cancer in female RA patients,
123 although another study reported an increased risk for cervical cancer. Kim, et al.
25 studied the risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory disease, including inflammatory bowel disease, psoriasis, RA, and systemic lupus erythematosus. Hazard ratios [HRs (95% CIs)] for high-grade cervical dysplasia and cervical cancer were 1.49 (95% CI: 1.11–2.00) in RA patients. Wadstrom, et al.
26 reported that biologic-naïve women with RA were at greater risk of cervical intraepithelial neoplasia (CIN) grade 1 (HR 1.53, CI: 1.23–1.89) and CIN 2–3 (HR 1.39, CI: 1.16–1.66), but not of invasive cervical cancer (HR 1.09, CI: 0.71–1.65), compared with the general population. RA patients are more likely to have persistent human papillomavirus (HPV) due to poor immune competence, which increases the risk of cervical cancer. Increased cervical cancer incidence may also be associated with systemic inflammation and immunosuppressant or the use of steroid. Although the efficacy of HPV vaccine has not been proven in patients with RA, young women with RA should consider a HPV vaccination, because the HPV vaccination is highly effective in preventing cervical cancer in the general population.
252627
This study has both limitations and strengths. Because this study was a retrospective observational study in a single center, only limited information is available and the sample size is small. For example, we could not evaluate the relationship between cancer development and RA disease activity. However, information on the diagnosis and treatment period of RA, cancer diagnosis date, and information on the malignancy site has high accuracy. Because the enrolled patients were diagnosed with RA and underwent DMARDs therapy at our hospital, the date of cancer diagnosis and the cancer site were confirmed on records of the Korean Central Cancer Registry and national death certificates along with the medical chart review. In addition, some detailed information about cancer patients was also available through a chart review, and the presence of smoking history or an underlying lung disease was also confirmed in patients with lung cancer.
Previous studies on the development of cancer in patients with RA have been conducted in several countries. This is the first study to compare the incidence of cancer among patients with RA to those of the general population in Korea. The results of this study are expected to be used as the baseline assessment of malignancy among Korean RA patients.
In conclusion, the overall incidence of cancers in Korean RA patients was increased in both male and female patients. The incidences of lung cancer and leukemia were increased in male RA patients, and the incidences of thyroid cancer, cervical cancer, non-Hodgkin's lymphoma, and gallbladder cancer were increased in female RA patients.
Go to :

 ± 1.96×0.5)2/expected number of cancer cases.14
± 1.96×0.5)2/expected number of cancer cases.14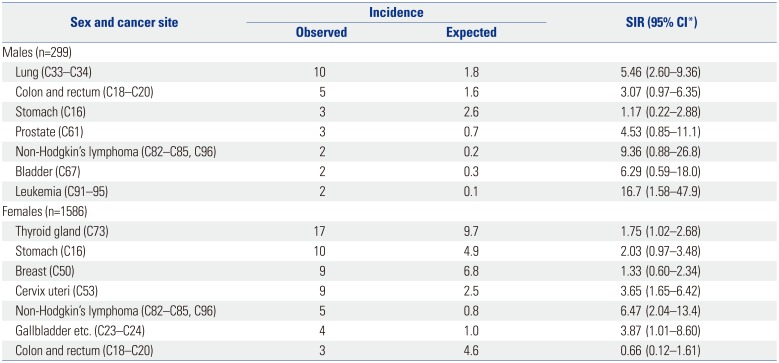
 ± 1.96×0.5)2/expected number of cancer cases.14
± 1.96×0.5)2/expected number of cancer cases.14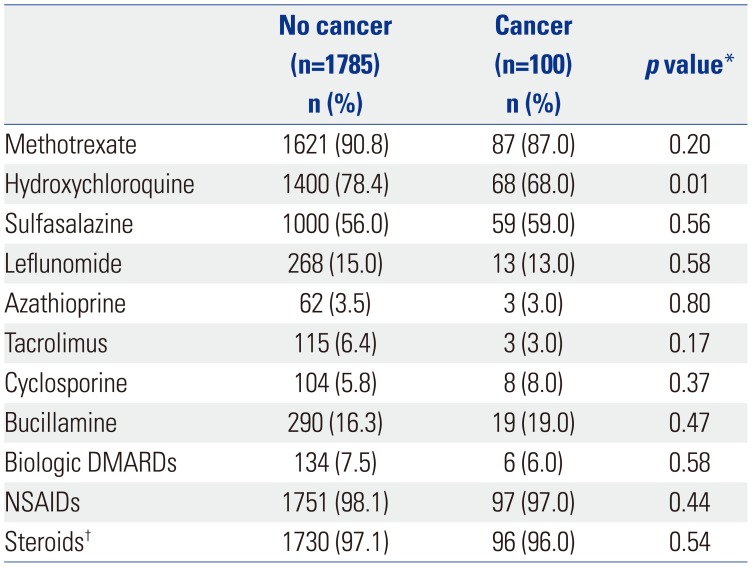




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download