INTRODUCTION
Drug-induced adverse events (AEs) can extend hospitalization, cause death,
1 and lead to physical or mental damage of patients.
23 Pharmacovigilance has been defined by the World Health Organization (WHO) as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other possible drug-related problems.”
4 It is important to detect adverse drug reactions (ADRs) over time, in order to use drugs more safely for the treatment of chronic diseases.
5 A signal in pharmacovigilance refers to an essential hypothesis of the risks due to medicines with data and arguments supporting it. This information is derived from data from one or more of many possible sources that are defined by the World Health Organization Uppsala Monitoring Centre (WHO-UMC).
6
Approximately 10% of Koreans over 30 years of age have diabetes mellitus,
7 which was the sixth most common underlying cause of death from cardiovascular AEs in 2016.
8 Common cardiovascular complications in patients with diabetes include coronary artery disease, stroke, peripheral arterial disease, cardiomyopathy, and heart failure.
9 Although most studies have shown that dipeptidyl peptidase-4 (DPP4) inhibitors reduce cardiovascular AEs, reports of unexpected heart failure raised questions about the cardiovascular safety of DPP-4 inhibitors.
1011 In a case-control study of saxagliptin, the rate of hospitalization due to heart failure was 3.5% in saxagliptin group and 2.8% in placebo group [hazard ratio, 1.27; 95% confidence interval (CI), 1.07 to 1.51;
p=0.007].
12 In a meta-analysis of sitagliptin, patients with type 2 diabetes and cardiovascular AEs did not display an increased risk of major cardiovascular disease or hospitalization due to heart failure when sitagliptin was used in combination with conventional antidiabetic drugs.
13 One study on sitagliptin found an increased rate of hospitalization for heart failure,
14 but no significant risk of death was observed in a cohort study.
15 In this study, hospitalization due to heart failure in sitagliptin group showed a 10% increased risk compared to control group (hazard ratio, 1.09; 95% CI, 1.06 to 1.11;
p<0.001). A meta-analysis of AEs of DPP-4 inhibitors showed that they did not increase the risk of cardiovascular disease and stroke in patients with diabetes, but that their long-term use increased the risk of heart failure by 15.8% (relative risk, 1.158; 95% CI, 1.011 to 1.326;
p=0.034).
16 A study of the association of alogliptin with acute coronary disease did not show a higher incidence of major cardiovascular AEs in patients who took alogliptin compared to those who were given a placebo.
17 Therefore, the results of studies that have investigated the association of DPP-4 inhibitors with cardiovascular risk have varied, and the safety of DPP-4 inhibitors remains unconfirmed.
Despite previous randomized controlled trials and studies from North America and Europe, race-related characteristics regarding cardiovascular events still exist. To date, studies on the association of DPP-4 inhibitors with cardiovascular risk have been conducted mainly in foreign countries, so there are no safety data from a Korean population; therefore, studies and signal analyses based on domestic data are needed. The purpose of this study was to analyze the Korea Institute of Drug Safety & Risk Management-Korea Adverse Event Reporting System Database (KIDS-KD) and detect signals that represent the association between DPP-4 inhibitors and cardiovascular AEs.
MATERIALS AND METHODS
Database and study drug
This study used data on spontaneous AEs reports about DPP-4 inhibitors and other oral antidiabetic drugs from KIDS-KD. The data were collected between January 2008, when DPP-4 inhibitors were first marketed in Korea, and December 2016; the controls were other oral antidiabetic drugs. This study was conducted under prior approval for using data collected by the Korea Adverse Event Reporting System (KAERS), which was obtained in June 2017.
In this study, we screened data on ADRs reported in KAERS, while filtering out input errors and logical errors, and then cleansed the data to provide a uniform code for drug and AE information. The provided KIDS-KD included basic information, side effect information, drug information, reporter information, causality assessment information, serious unexpected ADR information, medical history information, and group information; and we analyzed basic information, drug information, reporter information, and causality assessment information by referring to related manuals.
18
Active ingredients of antidiabetic drugs were classified by using the Anatomical Therapeutic Chemical code, which is managed by the World Health Organization Collaborating Centre for Drug Statistics Methodology. To define suspected AEs, preferred terms (PTs) and included terms (ITs) codes, which are two out of four components of the World Health Organization Adverse Reaction Terminology (WHO-ART) 092 version were used. WHO-ART is a system that was developed for the purpose of reasonably coding AEs terms in pharmaceutical regulatory agencies and pharmaceutical companies around the world. It comprises hierarchical structures with 32 system-organ classes (SOCs), 189 high level terms (HLTs), 2178 PTs, and 5813 ITs. We used signal analysis to identify ADRs after the use of DPP-4 inhibitors.
19
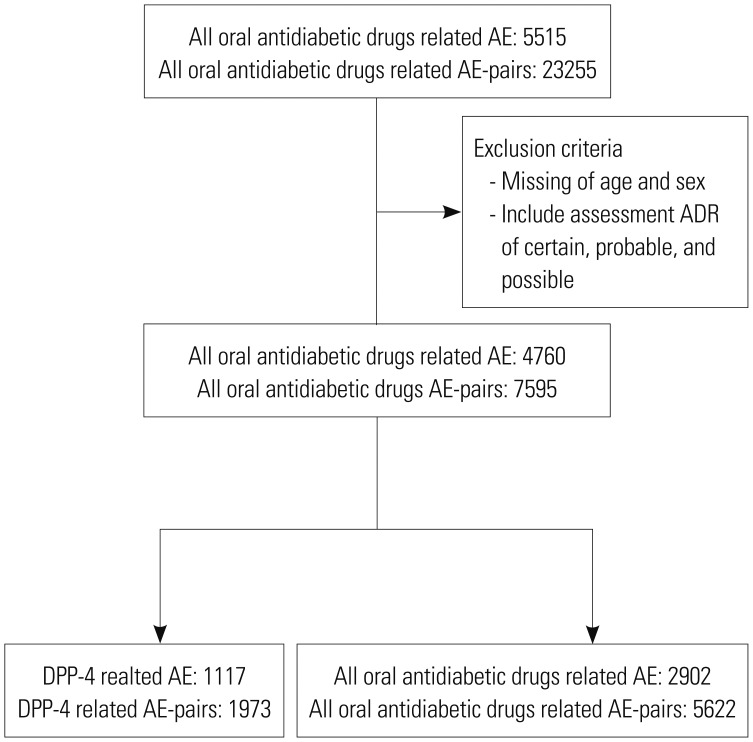
From 5515 cases out of 15237 reports, we limited the cases to those with causality assessment information that were evaluated as definite, probable, and possible. We also excluded patients for whom sex and age data were missing. The final data included 1117 cases of DPP-4 inhibitors and 2902 of other oral antidiabetic drugs (
Fig. 1).
DPP-4 inhibitors and other oral antidiabetic drugs
The study drugs were oral antidiabetic drugs, classified as sulfonylureas, meglitinides, biguanides, thiazolidinediones, alpha-glucosidase inhibitors, and DPP-4 inhibitors, which have been prescribed in Korea. We excluded medications that were not orally administered, such as glucagon-like peptide-1 agonists and insulins. We defined users of DPP-4 inhibitors as those who were treated with a single DPP-4 inhibitor or combined administration, including DPP-4 inhibitors.
Definition of cardiovascular AEs and all other ADRs
Cardiovascular AEs consisted of the following SOC codes from WHO-ART: 1010 (general cardiovascular disorders), 1020 (myo-, endo-, pericardial, and valve disorders), 1030 (heart rate and rhythm disorders), and 1040 [vascular (extracardiac) disorder]. Furthermore, we investigated the frequency of AE-pairs associated with DPP-4 inhibitors and all other oral antidiabetic drugs through PT codes, and grouped AEs by SOC.
Data mining indices
Data mining refers to the determination of signal using proportional reporting ratios (PRR), reporting odds ratio (ROR), and other parameters,
20 and has been applied for drug monitoring since the late 1990s. The term “signal” refers to information that is obtained by conducting data mining with post-marketing spontaneous reporting data, and specifically refers to information that is unlikely to exclude the possibility of a causal association with drugs.
21 Signal was confirmed by comparison with precautionary notes of the licensed drug.
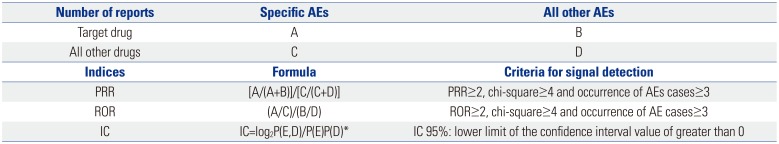
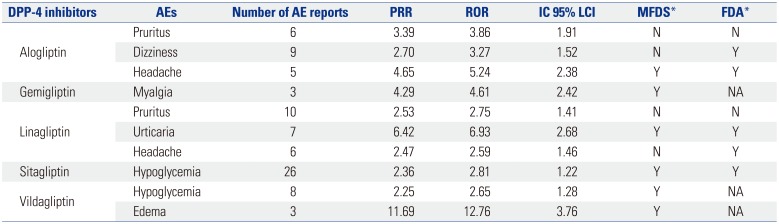
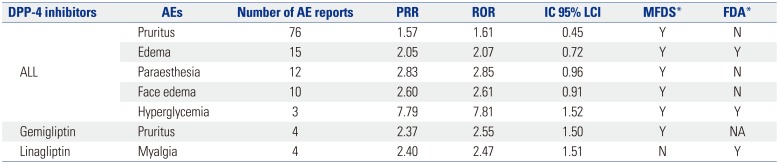
Data mining methods using ROR, PRR, and Bayesian confidence propagation neural networks of information components (IC) were applied to signal index calculations (
Table 1). Statistic PRR was obtained from the division of specific AE fraction of a specific drug reporting by specific AE fraction of another drug reporting. The criteria were PRR ≥2, chi-square ≥4, and number of AEs ≥3. ROR is defined as the odds of occurrence of a specific AE in a patient who is exposed to a specific drug, divided by the odds of occurrence of an AE specific to the other drug; ROR ≥2, chi-square ≥4, and number of AEs ≥3 were the determination criteria. The IC value was a log of the probability that drug use and specific AE coexist, divided by the product of probability of drug use and probability of occurrence of a specific AE under the assumption that drug use and the occurrence of certain AEs were independent of each other. The criteria for determining the IC value was that the 95% CI, and that their lower limit was greater than 0. ADRs satisfying all three criteria were defined as signals.
The calculation for PRR, ROR, and IC used two-by two-contingency tables of reported event counts for specific drug and other drugs, which comprised DPP-4 inhibitors, all other oral antidiabetic drugs in the rows and cardiovascular disorders as the above definition, and all AEs in columns. In this study, three data mining algorithms of PRR, ROR, and IC were used to assess whether the signals of specific drugs were significant (
Table 1).
Drug label information of Korea Ministry of Food and Drug Safety and U.S. Food and Drug Administration
Statistical analysis
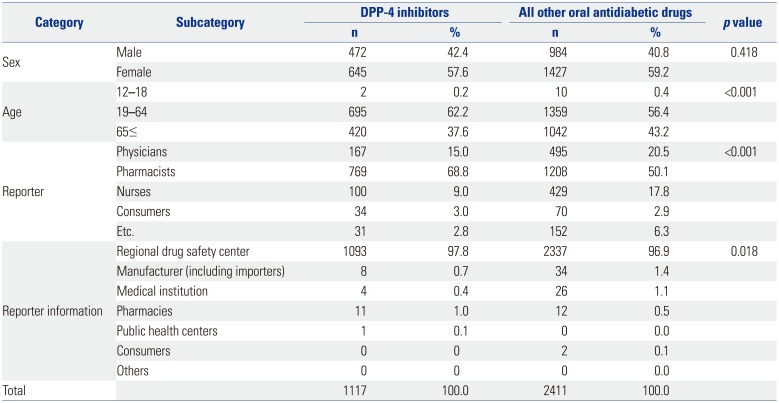
Basic demographic characteristics about age, sex, reporter, and reporter information were analyzed. Age groups were divided into three groups of 12–18, 19–64, and over 65 years. Reporters included physicians, pharmacists, and nurses, and the reported information contained regional drug safety center, manufactures and importers, medical institutions, pharmacies, public health centers, and consumers.
Descriptive statistics were used to analyze basic information, and the data coded by PT and IT were classified into SOC system to accurately classify the oral antidiabetic drugs. We conducted a chi-square test for categorical variables and a t-test or exact test for continuous variables. Data-mining indices were explained above.
All statistical analyses were computed by using SAS statistical application program (Version 9.4, SAS Institute Inc., Cary, NC, USA). The study protocol was approved by Sungkyunkwan University's Institutional Review Board (IRB No. 2016-09-012). Informed consent was waived by the board.
DISCUSSION
As life expectancy increases and diseases become chronic, the long-term use of medicine results in increased side effects. Prospective studies of drug-induced adverse reactions have been under way for approximately 35 years, and the Boston Collaborative Drug Surveillance Program is a large-scale study of the degree of drug-induced hypersensitivity. A meta-analysis of 39 prospective studies that were performed in U.S. hospitals from 1966 to 1996 found 75 to 106000 deaths due to AEs per year.
22 According to a study by Lazarou, et al.
23 regarding AE incidences that require hospitalization, AEs are between the sixth and eighth leading cause of death in U.S. hospitals: 6.7% of admitted patients experienced serious drug side effects, and 0.32% of these patients died.
We need to confirm the safety of antidiabetic drugs, since the prevalence of diabetes mellitus is continuously increasing and the disease requires long-term use of medications. However, according to the Korean Diabetes Association's Diabetes Care Guideline for 2015, some DPP-4 inhibitors are stated to have uncertain cardiovascular safety during long-term use. Therefore, a study of the association between DPP-4 inhibitor and cardiovascular AEs was needed in Korea, and we performed an analysis with KIDS-KD to detect post-marketing surveillance (PMS) signals over 9 years.
The signals that satisfied all three indices of PRR, ROR, and IC for DPP-4 inhibitors were itching, dizziness, headache, myalgia, urticaria, hypoglycemia, and edema. These signals were not related to cardiovascular AEs, and hypoglycemia was most common AE. Itching and dizziness and itching and headache, which were associated with linagliptin, were not labeled ADRs in MFDS.
In a previous study, significant associations between DPP-4 inhibitors and cardiovascular AEs were found. However, this study did not identify any significant associations between DPP-4 inhibitors and cardiovascular AEs. We reviewed the differences between this study and other previous studies that investigated the association between heart failure and DPP-4 inhibitors using the FDA Adverse Event Reporting System data using similar methods.
One previous study used spontaneous reporting data of DPP-4 inhibitors and rosiglitazone, and found that saxagliptin and sitagliptin were associated with heart failure.
24 Unlike the results of our study, significant results were obtained regarding the association between DPP-4 inhibitors and cardiovascular risk. In the study of Raschi, et al.,
24 42198 cases of AEs were reported across all antidiabetic drugs; of these, 8963 were attributed to DPP-4 inhibitors. The AEs that were reported in our study included 4019 cases across all antidiabetic drugs and 1117 cases related to DPP-4 inhibitors. The DPP-4 inhibitor data set ranged from 1/8 to 1/10 of that for all antidiabetic drugs. In addition, the study showed that the significant association with cardiovascular AEs was due to the AEs of sitagliptin and saxagliptin, which were used as DPP-4 inhibitors in 90% of the cases. In contrast, in a study that was based on domestic data, sitagliptin and saxagliptin accounted for 45.8% of the reported AEs (453 and 59 cases, respectively, out of 1117 AEs due to DPP-4 inhibitors). In a literature review, four out of six studies related to sitagliptin and saxagliptin as DPP-4 inhibitors were associated with cardiovascular AEs, suggesting that sitagliptin and saxagliptin are more closely related to cardiovascular AEs than other DPP-4 inhibitors. Based on these results, we conclude that the ratio of sitagliptin and saxagliptin among all of the DPP-4 inhibitors that were investigated in our study is lower than that of previous overseas studies.
However, this study had some advantages, which can be summarized in three ways. Signal detection was used to find an adverse reaction signal that can be causally related to drugs through data mining, and does not alone confirm the causality with the drug.
25 Therefore, a well-designed pharmacokinetic study should determine causality. However, this study is important as a drug pharmacoepidemic study that provides basic data about DPP-4 inhibitor related ADRs. Second, by using data of KIDS-KD from KAERS, we analyzed all types of DPP-4 inhibitors from PMS in comparison with other oral antidiabetic drugs. Third, this study investigated the association between cardiovascular AEs and DPP-4 inhibitors, and only a few studies which investigated this topic were performed in Korea.
This study also had some limitations. First, DPP-4 inhibitors have been on the market since 2008, and the duration of the study from 2008 to 2016 was insufficient to study the association between DPP-4 inhibitors and cardiovascular AEs, given the timescale of cardiovascular events. Therefore, further long-term follow-up studies are needed in the future. Second, to supplement the limitations of KIDS-KD data, it is necessary to perform a signal analysis that can produce statistically accurate results across large-scale data sources, such as that of the National Health Insurance Corporation. Third, a comparative study of DPP-4 inhibitors and cardiovascular AEs between the consumers and medical experts will be necessary if the future consumer report is sufficient, as the number of studies that compare consumer and medical expert reports are increasing worldwide. However, since the perception rates of the general public and consumers are low, the rate of continued reports on drug side effects will be lower. In fact, in the first half of 2008, the rate of pharmaceutical reports was 45.5%, pharmaceuticals was 51.8%, pharmacies was 2.0%, and consumers was 0.4%; and for other public health centers, government offices, associations, and consumer organization, the rate of reports was 0.3%.
26 This was consistent with the finding that 8.3% (±2.53%) of the public perception rate of spontaneous drug side effects were reported.
27
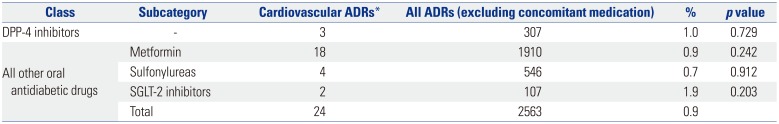
In this study, DPP-4 related cardiovascular AEs included three cases of palpitation after administration of sitaglipitin and gemigliptin. Our findings were underpowered, since the observed number of cases of cardiovascular events was small. We found that there was no association between the use of DPP-4 inhibitors and cardiovascular AEs, and that cardiovascular AEs were detected after taking DPP-4 inhibitors in only three (1.0%) out of 307 cases.
This result was similar to that obtained when using all other oral diabetic drugs with a longer use experience than DPP-4 inhibitors, and was not statistically significant. It is likely attributable to the limitations associated with spontaneous reporters of AEs. Most of the reporters were pharmacists (68.8%), with doctors and nurses comprising 15.0% and 9.0% of reporters, respectively. Since serious cardiovascular AEs are considered to be complications of diabetes mellitus rather than ADRs, which were serious in this study, the contribution to cardiovascular diseases by diabetic drugs, including DPP-4 inhibitors, is likely to be underestimated.
Although no significant cardiovascular events were observed in this study, some differences in AEs compared to the label information of FDA were found. Although these findings were not non-cardiovascular AEs, they showed race-related characteristics. Therefore, further studies comparing data across multiple countries are required. In addition, AEs will need to be continuously reported by physicians and other healthcare providers with respect to their patient complaints, even after they have been reported by the pharmaceutical company during PMS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download