Abstract
Materials and Methods
Results
Figures and Tables
Fig. 1
miRNA-206 expression is inversely correlated with PAX3 or MET mRNA expression in osteosarcoma and adjacent tissue specimens. (A–F) mRNA expression levels of miR-206, MET mRNA, and PAX3 mRNA in 25 pairs of OS and adjacent tissue specimens. (G and H) Pearson correlation analysis determining the significance of correlation between miR-206 expression and PAX3 or MET mRNA expression. (I and J) Protein levels of c-Met and Pax3 that are encoded by MET and PAX3 gene in 25 pairs of OS and adjacent tissue specimens. OS, osteosarcoma.

Fig. 2
miR-206 interacts with PAX3 or MET mRNA in OS cells. (A) AGO2-RIP assay determining the association between AGO2 protein and mRNA of indicated genes in OS cells after transfection with different miRNA mimics. (B) miRNA pulldown assay determining the association between miR-206 and mRNA of indicated genes in OS cells. miR-NC transfected group was used as control for significance test. OS, osteosarcoma.

Fig. 3
miR-206 reduces PAX3 and MET expression, as well as Akt1 and Erk1/2 activation. (A–C) RT-qPCR evaluating miR-206, PAX3 mRNA and MET mRNA in OS cells after indicated lentiviral transduction. (D–H) Western blot detecting the protein expression levels of PAX3 and MET, as well as Akt1 Ser473 and Erk1/2 total threonine phosphorylation, in OS cells after indicated transduction. NT was used as control for significance test. OS, osteosarcoma; NT, non-transduced group.

Fig. 4
miR-206 reduces OS cell proliferation while promoting apoptosis in vitro. miR-206 overexpression or knockdown in OS cells in vitro was achieved by lentiviral transduction. (A) CCK-8 cell viability assay evaluating overall cell growth in vitro. (B) Western blot evaluating the protein expression levels of the cell proliferation marker PCNA. (C) Flow cytometry detection of cell apoptosis. (D) Western blot evaluating the activation of pro-apoptotic protein Caspase-3. (E) Representative results of Western blots. NT was used as a control for significance test. *p<0.01, †p<0.001. OS, osteosarcoma; NT, non-transduced group.

Fig. 5
miR-206 reduces OS cell migration (A) and invasion (B) in vitro. miR-206 overexpression or knockdown in OS cells in vitro was achieved by lentiviral transduction. (C) Representative results of cell migration and invasion assays. NT was used as a control for significance test. OS, osteosarcoma; NT, non-transduced group.

Fig. 6
miR-206 reduces OS cell malignancy in vitro by targeting PAX3 and MET. miR-206 overexpressing OS cells were transfected with PAX3 or MET OE plasmids or treated with HGF before assay. (A) CCK-8 cell viability assay evaluating the overall cell growth in vitro. (B) Flow cytometry detection cell apoptosis. (C and D) Cell invasion and migration assay evaluating cell metastasis in vitro. (E) Representative results of cell migration and invasion assay. WT OS cells were used as a control for significance test. *p<0.01, †p<0.001. OS, osteosarcoma; OE, overexpressing; WT, wild-type, HGF, hepatocyte growth factor; NC, negative control.
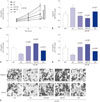
Fig. 7
miR-206 reduces PAX3 and MET gene expression and IP3K-AKT, MAPK-ERK signaling in OS cells. OS cells were treated as indicated in Fig. 6 before Western blot assay (A), Western blot detecting the protein expression levels of Pax3 or c-Met in OS cells after different treatment. (B and C) Western blot detecting Akt1 Ser473 or Erk1/2 total threonine phosphorylation in OS cells after different treatments. (D) Representative Western blot results. WT OS cells were used as a control for significance test.. OS, osteosarcoma; WT, wild-type.

Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Fang-Biao Zhan.
Data curation: Xian-Wei Zhang, Shi-Long Feng.
Formal analysis: Fang-Biao Zhan, Xian-Wei Zhang.
Funding acquisition: Xian-Wei Zhang.
Investigation: Fang-Biao Zhan, Shi-Long Feng.
Methodology: Xian-Wei Zhang, Shi-Long Feng, Jun Cheng.
Project administration: Fang-Biao Zhan.
Resources: Jun Cheng, Bo Li.
Software: Jun Cheng, You Zhang.
Validation: Li-Zhong Xie, Qian-Rong Deng.
Writing—original draft: You Zhang.
Writing—review & editing: Bo Li, Li-Zhong Xie, Qian-Rong Deng.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download