Abstract
Purpose
To evaluate the influence of axial length on the recurrence of wet age-related macular degeneration (AMD) after an-ti-vascular endothelial growth factor therapy.
Methods
A retrospective review of the medical records for 45 eyes of 45 patients, who were diagnosed with neovascular AMD and treated with three ranibizumab injections per month, was performed. Axial length was compared between eyes with (recurrence group) and without (no recurrence group) recurrence of fluid during a 12-month follow-up period. In eyes with recurrence, the association between axial length and the time between the third injection and the first recurrence was also evaluated.
Results
The axial length was measured at a mean of 20.6 ± 10.1 months after the diagnosis of neovascular AMD. The mean axial length at that time was 23.33 ± 0.90 mm. The mean axial length was 23.29 ± 0.96 mm in the recurrence group (n = 30) and 23.40 ± 0.79 mm in the no-recurrence group (n = 15). There was no difference in the axial length between the two groups (p = 0.709). In the recurrence group, the period between the third injection and the first recurrence was not associated with axial length (p =0.582).
Conclusions
There was no significant difference in axial length between eyes with and without recurrence after initial treatment for wet AMD. In addition, the time to first recurrence was not significantly associated with axial length. Because the present study was retrospective and the sample size was small, further prospective studies with a better design are needed to more accurately assess the influence of axial length.
Go to : 
References
1. Park SJ, Kwon KE, Choi NK, et al. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015; 122:2063–70.e1.
2. Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122:477–85.
3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

4. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

5. Kang S, Cho WK, Roh YJ. The efficacy of ranibizumab for abdominal neovascularization in age-related macular degeneration. J Korean Ophthalmol Soc. 2009; 50:725–30.
6. Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular abdominal of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012; 154:682–6.e2.
7. Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics abdominal a single intravitreal injection of 1.5 mg versus 3.0 mg of abdominal in humans. Retina. 2011; 31:1877–84.
8. Fauser S, Muether PS. Clinical correlation to differences in abdominal and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol. 2016; 100:1494–8.
9. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal abdominal (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.
10. Kim JH, Chang YS, Lee DW, et al. Incidence and timing of the first recurrence in neovascular age-related macular degeneration: abdominal between ranibizumab and aflibercept. J Ocul Pharmacol Ther. 2017; 33:445–51.
11. Teichmann KD. Intravitreal injections: does globe size matter? J Cataract Refract Surg. 2002; 28:1886–9.

12. Krohne TU, Muether PS, Stratmann NK, et al. Influence of ocular volume and lens status on pharmacokinetics and duration of action of intravitreal vascular endothelial growth factor inhibitors. Retina. 2015; 35:69–74.

13. Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016; 100:1623–8.

14. Horster R, Ristau T, Sadda SR, Liakopoulos S. Individual abdominal intervals after anti-VEGF therapy for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2011; 249:645–52.
15. Kuroda Y, Yamashiro K, Miyake M, et al. Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: a retrospective cohort study. Ophthalmology. 2015; 122:2303–10.
16. Nagra M, Gilmartin B, Logan NS. Estimation of ocular volume from axial length. Br J Ophthalmol. 2014; 98:1697–701.

17. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous abdominal, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–11.
18. Lee D, Jeong S, Moon J, et al. Analysis of efficacy of intravitreal aflibercept according to subfoveal choroidal thickness in abdominal choroidal vasculopathy. J Korean Ophthalmol Soc. 2016; 57:1577–85.
19. Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular abdominal and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015; 56:1909–15.
20. Kim JH, Chang YS, Kim JW, et al. Prevalence of subtypes of abdominal pseudodrusen in newly diagnosed exudative age-related abdominal degeneration and polypoidal choroidal vasculopathy in Korean patients. Retina. 2015; 35:2604–12.
21. Ma L, Li Z, Liu K, et al. Association of genetic variants with abdominal choroidal vasculopathy: a systematic review and updated meta-analysis. Ophthalmology. 2015; 122:1854–65.
22. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal abdominal and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010; 29:19–29.
23. Lee WK, Baek J, Dansingani KK, et al. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or sub-normal subfoveal choroidal thickness. Retina. 2016; 36(Suppl 1):S73–82.

24. Inoue M, Yamane S, Sato S, et al. Comparison of time to reabdominal and visual function between ranibizumab and aflibercept in age-related macular degeneration. Am J Ophthalmol. 2016; 169:95–103.
25. Han X, Guo X, Lee PY, et al. Six-year changes in refraction and abdominal ocular biometric factors in an adult Chinese population. PLoS One. 2017; 12:e0183364.
26. Kymionis GD, Giarmoukakis A, Apostolidi IK, et al. Optical abdominal derived axial length measurements following intravitreal an-ti-vascular endothelial growth factor treatment for macular edema. Semin Ophthalmol. 2018; 33:488–91.
Go to : 
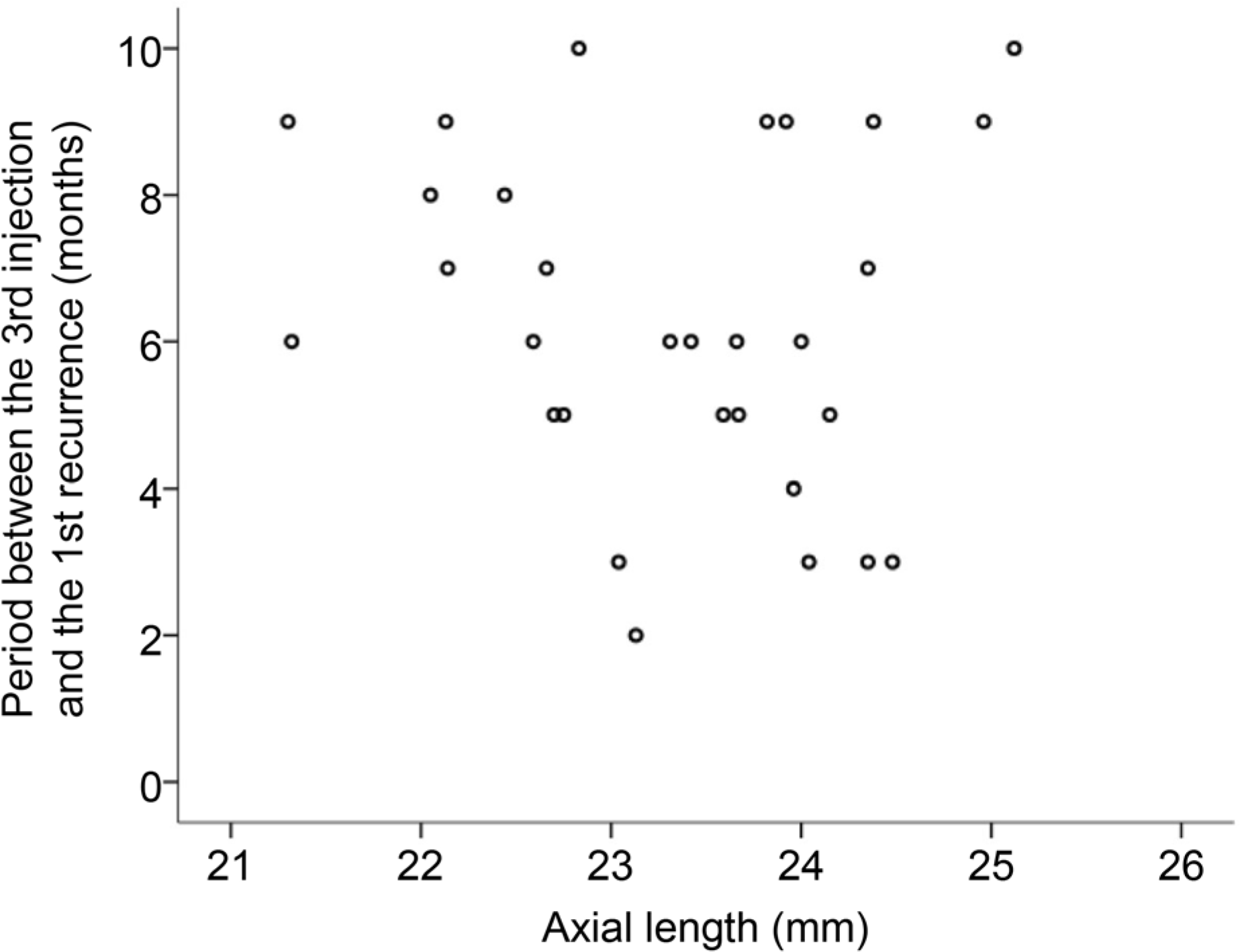 | Figure 1.A scatter plot showing association between axial length and the interval between the initial loading phase and the recurrence (n = 30). There was no significant association between the two factors (p = 0.582). |
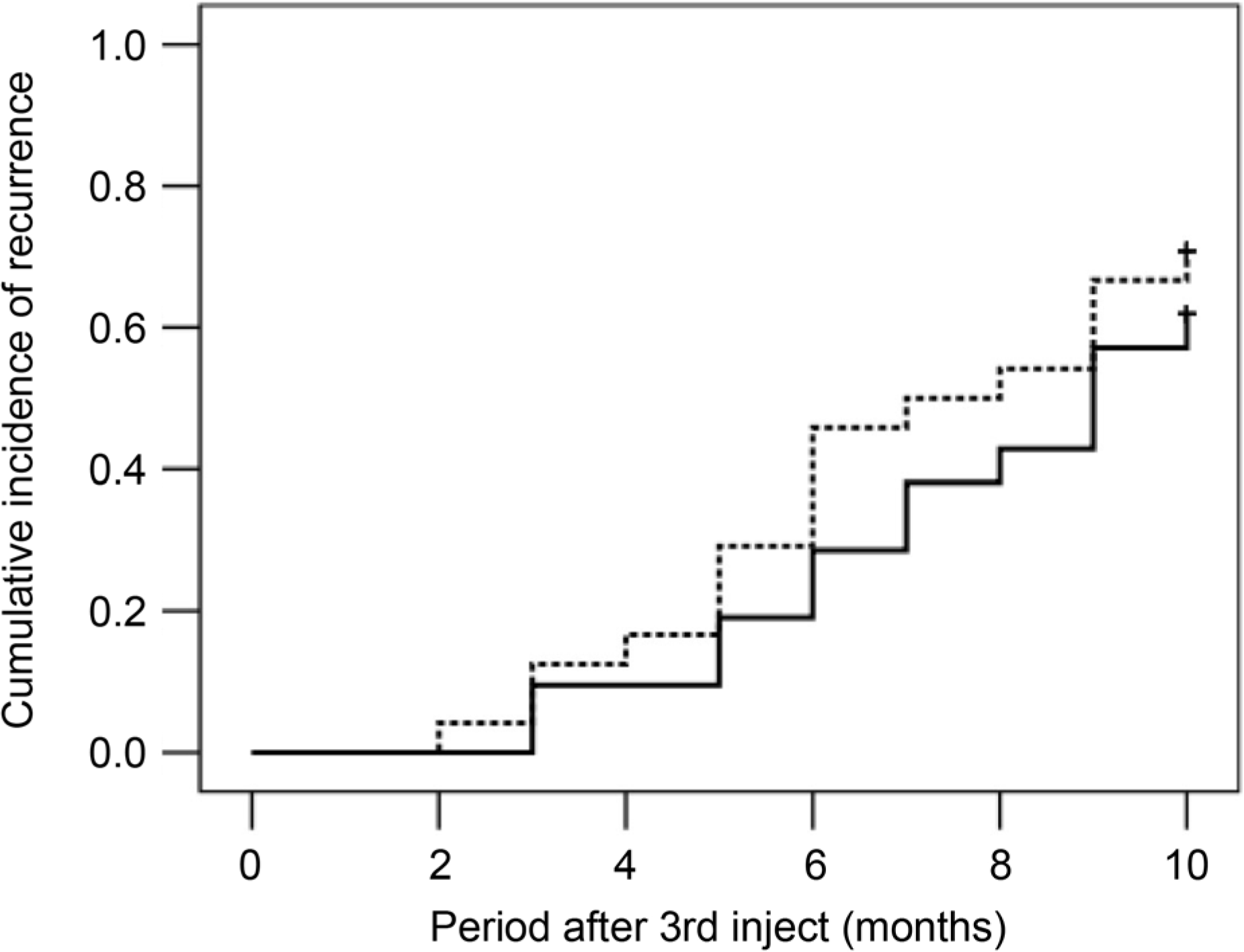 | Figure 2.A Kaplan-Meier graph showing cumulative incidence of recurrence, when divided into two groups, according to axial length. There was no significant difference in the recurrence between the short axial length group (n = 21, solid line) and long axial length group (n = 24, dotted line) (p = 0.409). |
Table 1.
Baseline characteristics of 45 patients (45 eyes) who were diagnosed with neovascular age-related macular degeneration
Table 2.
Comparison of axial length and other characteristics between patients with and without recurrence during the 12 months fol-low-up period
| Characteristic | With recurrence (n = 30) | Without recurrence (n = 15) | p-value |
|---|---|---|---|
| Axial length (median value, mm) | 23.29 ± 0.96 (23.37) | 23.40 ± 0.79 (23.26) | 0.709* |
| Age (years) | 76.3 ± 5.7 (76.0) | 75.7 ± 5.3 (77.0) | 0.743* |
| Sex | 0.671† | ||
| Men | 16 (53.3) | 9 (60.0) | |
| Women | 14 (46.7) | 6 (40.0) | |
| Hypertension | 17 (56.7) | 11 (73.3) | 0.277† |
| Diabetes mellitus | 6 (20.0) | 2 (13.3) | 0.699‡ |
| Types of neovascular AMD | 1.000‡ | ||
| Typical neovascular AMD + RAP | 15 (50.0) | 8 (53.3) | |
| Polypoidal choroidal vasculopathy | 10 (33.3) | 4 (26.7) | |
| Best-corrected visual acuity (logMAR, median value) | 0.61 ± 0.29 (0.40) | 0.72 ± 0.38 (0.70) | 0.339* |
Analysis regarding types of neovascular AMD was performed based on 37 eyes with available indocyanine-green angiography result. Values are presented as the mean ± standard deviation or number (%). AMD = age-related macular degeneration; RAP = retinal angiomatous proliferation; logMAR = logarithm of the minimal angle of resolution.
Table 3.
Comparison of axial length betw without recurrence when divided into tw the subtypes of neovascular age-related ween patients with and o groups, according to macular degeneration
| Characteristic | Axial length |
|---|---|
| Typical neovascular AMD + RAP (n = 23) | |
| With recurrence (n = 15) (median value) | 23.25 ± 1.21 (23.76) |
| Without recurrence (n = 8) (median value) | 23.49 ± 0.72 (23.19) |
| p-value* | 0.636 |
| Polypoidal choroidal vasculopathy (n = 14) | |
| With recurrence (n = 10) (median value) | 23.42 ± 0.66 (23.37) |
| Without recurrence (n = 4) (median value) | 23.95 ± 0.54 (24.01) |
| p-value* | 0.142 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download