1. Global Strategy for Diagnosis, Management, and Prevention of COPD-2016. Global Initiative for Chronic Obstructive Lung Disease. Accessed October 26, 2017. Available at:
http://goldcopd.org/.
2. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011; 365:1567–1575. PMID:
22029978.

3. Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010; 11:122. PMID:
20831787.

4. Daudey L, Peters JB, Molema J, Dekhuijzen PN, Prins JB, Heijdra YF, et al. Health status in COPD cannot be measured by the St George's Respiratory Questionnaire alone: an evaluation of the underlying concepts of this questionnaire. Respir Res. 2010; 11:98. PMID:
20649991.

5. Bergin C, Müller N, Nichols DM, Lillington G, Hogg JC, Mullen B, et al. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986; 133:541–546. PMID:
3963623.
6. Nakano Y, Sakai H, Muro S, Hirai T, Oku Y, Nishimura K, et al. Comparison of low attenuation areas on computed tomographic scans between inner and outer segments of the lung in patients with chronic obstructive pulmonary disease: incidence and contribution to lung function. Thorax. 1999; 54:384–389. PMID:
10212100.

7. Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008; 186:157–165. PMID:
18351420.

8. Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011; 261:274–228. PMID:
21788524.

9. Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009; 34:858–865. PMID:
19324952.

10. Kim SS, Jin GY, Li YZ, Lee JE, Shin HS. CT quantification of lungs and airways in normal Korean subjects. Korean J Radiol. 2017; 18:739–748. PMID:
28670169.

11. Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, et al. KOLD Study Group. Study design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort study. Tuberc Respir Dis (Seoul). 2014; 76:169–174. PMID:
24851130.

12. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338. PMID:
16055882.
13. Tweeddale PM, Merchant S, Leslie M, Alexander F, McHardy GJ. Short term variability in FEV1: relation to pretest activity, level of FEV1, and smoking habits. Thorax. 1984; 39:928–932. PMID:
6515598.

14. Herpel LB, Kanner RE, Lee SM, Fessler HE, Sciurba FC, Connett JE, et al. National Emphysema Treatment Trial Research Group. Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials. Am J Respir Crit Care Med. 2006; 173:1106–1113. PMID:
16497996.
15. Cho YH, Seo JB, Kim N, Lee HJ, Hwang HJ, Kim EY, et al. Comparison of a new integral-based half-band method for CT measurement of peripheral airways in COPD with a conventional full-width half-maximum method using both phantom and clinical CT images. J Comput Assist Tomogr. 2015; 39:428–436. PMID:
25700223.

16. Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012; 18:1711–1715. PMID:
23042237.

17. Kim EY, Seo JB, Lee HJ, Kim N, Lee E, Lee SM, et al. Detailed analysis of the density change on chest CT of COPD using non-rigid registration of inspiration/expiration CT scans. Eur Radiol. 2015; 25:541–549. PMID:
25218764.

18. Lee SM, Seo JB, Lee SM, Kim N, Oh SY, Oh YM. Optimal threshold of subtraction method for quantification of air-trapping on coregistered CT in COPD patients. Eur Radiol. 2016; 26:2184–2192. PMID:
26515547.

19. Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006; 100:1742–1752. PMID:
16549342.

20. Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000; 162(3 Pt 1):1102–1108. PMID:
10988137.
21. Nakano Y, Müller NL, King GG, Niimi A, Kalloger SE, Mishima M, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002; 122(6 Suppl):271S–275S.

22. Kim N, Seo JB, Song KS, Chae EJ, Kang SH. Semi-automatic measurement of the airway dimension by computed tomography using the full-with-half-maximum method: a study of the measurement accuracy according to the orientation of an artificial airway. Korean J Radiol. 2008; 9:236–242. PMID:
18525226.

23. Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006; 11:731–740. PMID:
17052301.

24. Pistolesi M, Camiciottoli G, Paoletti M, Marmai C, Lavorini F, Meoni E, et al. Identification of a predominant COPD phenotype in clinical practice. Respir Med. 2008; 102:367–376. PMID:
18248806.

25. Pennock BE, Rogers RM, McCaffree DR. Changes in measured spirometric indices. What is significant? Chest. 1981; 80:97–99. PMID:
7249720.
26. Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. ECLIPSE investigators. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008; 31:869–873. PMID:
18216052.



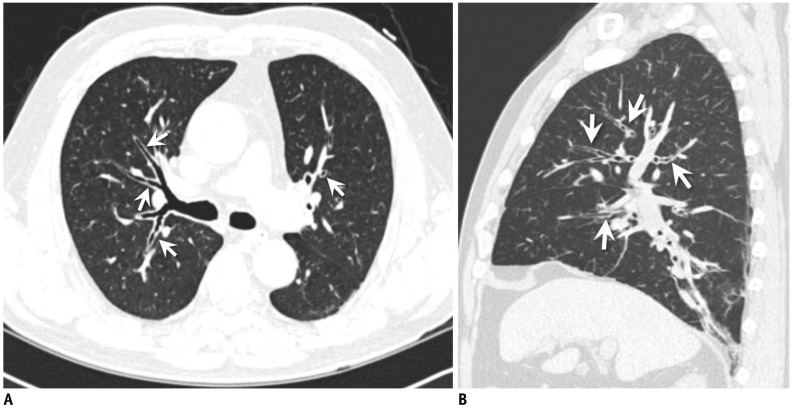
 ) was plotted against Pi of that airway. From regression line, standardized measure of airway wall thickness was predicted with Pi of 10 mm. Pi = internal perimeter
) was plotted against Pi of that airway. From regression line, standardized measure of airway wall thickness was predicted with Pi of 10 mm. Pi = internal perimeter





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download