1. Keller PJ, Drayer BP, Fram EK, Williams KD, Dumoulin CL, Souza SP. MR angiography with two-dimensional acquisition and three-dimensional display. Work in progress. Radiology. 1989; 173:527–532. PMID:
2798885.

2. Miyazaki M, Akahane M. Non-contrast enhanced MR angiography: established techniques. J Magn Reson Imaging. 2012; 35:1–19. PMID:
22173999.

3. Wheaton AJ, Miyazaki M. Non-contrast enhanced MR angiography: physical principles. J Magn Reson Imaging. 2012; 36:286–304. PMID:
22807222.

4. Laub GA. Time-of-flight method of MR angiography. Magn Reson Imaging Clin N Am. 1995; 3:391–398. PMID:
7584245.

5. Cho YD, Kim KM, Lee WJ, Sohn CH, Kang HS, Kim JE, et al. Time-of-flight magnetic resonance angiography for follow-up of coil embolization with enterprise stent for intracranial aneurysm: usefulness of source images. Korean J Radiol. 2014; 15:161–168. PMID:
24497808.

6. Papke K, Brassel F. Modern cross-sectional imaging in the diagnosis and follow-up of intracranial aneurysms. Eur Radiol. 2006; 16:2051–2066. PMID:
16416105.

7. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999; 42:952–962. PMID:
10542355.

8. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002; 47:1202–1210. PMID:
12111967.

9. Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007; 58:1182–1195. PMID:
17969013.

10. Donoho DL. Compressed sensing. IEEE Trans Inf Theory. 2006; 52:1289–1306.

11. Liang D, Liu B, Wang J, Ying L. Accelerating SENSE using compressed sensing. Magn Reson Med. 2009; 62:1574–1584. PMID:
19785017.

12. Stalder AF, Schmidt M, Quick HH, Schlamann M, Maderwald S, Schmitt P, et al. Highly undersampled contrast-enhanced MRA with iterative reconstruction: integration in a clinical setting. Magn Reson Med. 2015; 74:1652–1660. PMID:
25522299.

13. Rapacchi S, Han F, Natsuaki Y, Kroeker R, Plotnik A, Lehrman E, et al. High spatial and temporal resolution dynamic contrast-enhanced magnetic resonance angiography using compressed sensing with magnitude image subtraction. Magn Reson Med. 2014; 71:1771–1783. PMID:
23801456.

14. Hutter J, Grimm R, Forman C, Hornegger J, Schmitt P. Highly undersampled peripheral time-of-flight magnetic resonance angiography: optimized data acquisition and iterative image reconstruction. MAGMA. 2015; 28:437–446. PMID:
25605300.

15. Forman C, Piccini D, Grimm R, Hutter J, Hornegger J, Zenge MO. High-resolution 3D whole-heart coronary MRA: a study on the combination of data acquisition in multiple breath-holds and 1D residual respiratory motion compensation. MAGMA. 2014; 27:435–443. PMID:
24402560.

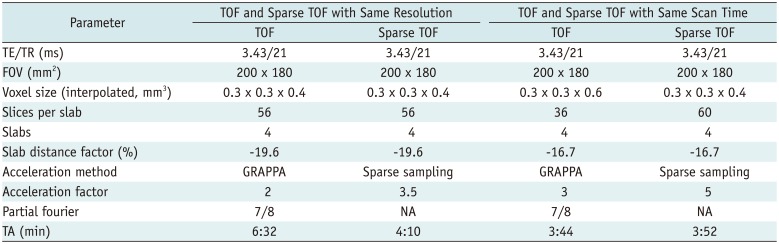
16. Natsuaki Y, Bi X, Zenge M, Speier P, Schmitt P, Laub G. Time-Of-Flight with sparse undersampling (TOFu): towards practical MR applications of the compressed sensing. Proc Intl Soc Mag Reson Med. 2014; 22:941.
17. Stalder AF, Natsuaki Y, Schmidt M, Bi X, Zenge MO, Nadar M, et al. Accelerating TOF MRA in clinical practice using sparse MRI with variable poisson density sampling. In : International Society for ISMRM Magnetic Resonance in Medicine: ONE community for Clinicians and scientists 23rd annual meeting; 2015 May 30-June 5; Toronto, Canada.
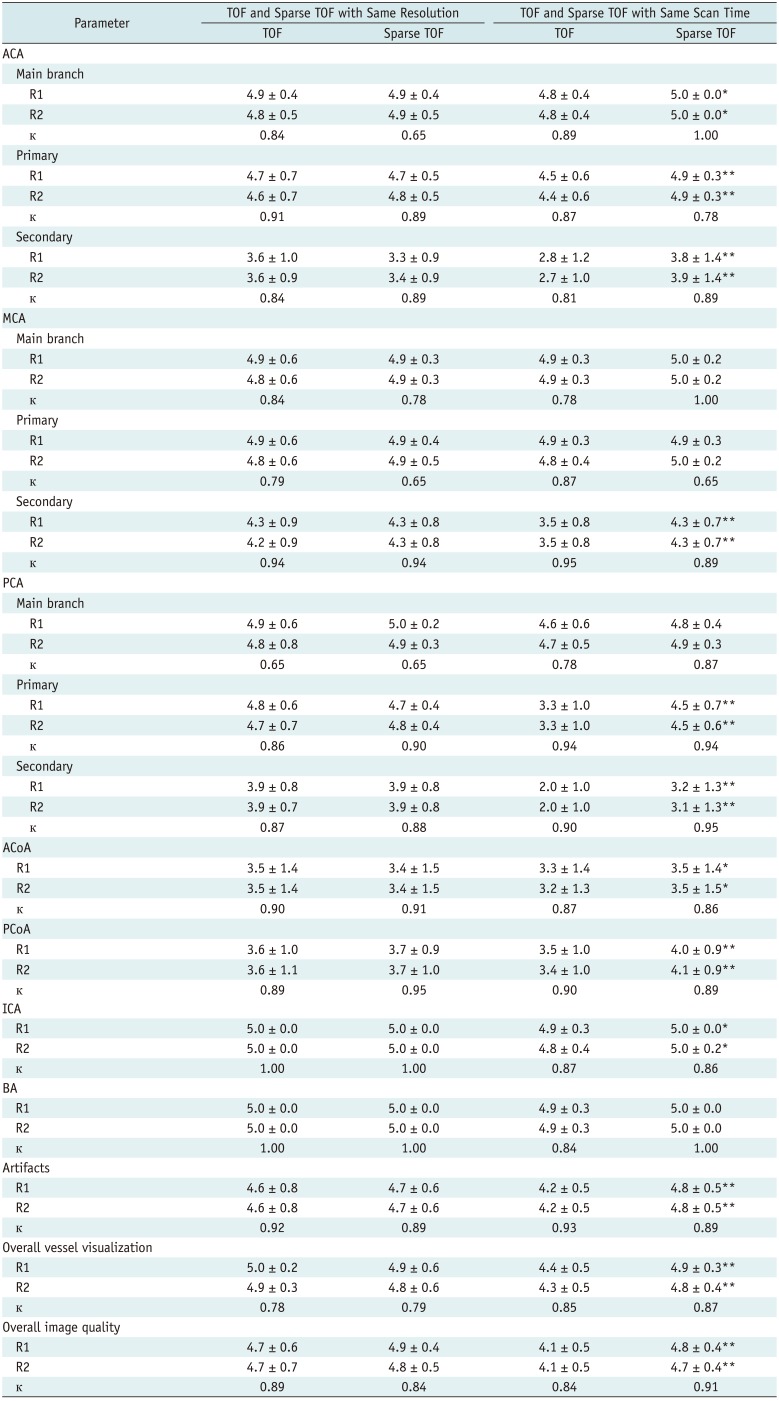
18. Fushimi Y, Okada T, Kikuchi T, Yamamoto A, Okada T, Yamamoto T, et al. Clinical evaluation of time-of-flight MR angiography with sparse undersampling and iterative reconstruction for cerebral aneurysms. NMR Biomed. 2017; 30:3774.

19. Yamamoto T, Fujimoto K, Okada T, Fushimi Y, Stalder AF, Natsuaki Y, et al. Time of Flight magnetic resonance angiography with sparse undersampling and iterative reconstruction: comparison with conventional parallel imaging for accelerated imaging. Invest Radiol. 2016; 51:372–378. PMID:
26561046.
20. Fushimi Y, Fujimoto K, Okada T, Yamamoto A, Tanaka T, Kikuchi T, et al. Compressed sensing 3-dimensional time-of-flight magnetic resonance angiography for cerebral aneurysms: optimization and evaluation. Invest Radiol. 2016; 51:228–235. PMID:
26606551.
21. Akasaka T, Fujimoto K, Yamamoto T, Okada T, Fushimi Y, Yamamoto A, et al. Optimization of regularization parameters in compressed sensing of magnetic resonance angiography: can statistical image metrics mimic radiologists' perception? PLoS One. 2016; 11:e0146548. PMID:
26744843.

22. Kayvanrad M, Lin A, Joshi R, Chiu J, Peters T. Diagnostic quality assessment of compressed sensing accelerated magnetic resonance neuroimaging. J Magn Reson Imaging. 2016; 44:433–444. PMID:
26777856.

23. Beck A, Teboulle M. A fast iterative shrinkage-Thresholding algorithm for linear inverse problems. SIAM J Imaging Sci. 2009; 2:183–202.

24. Wrede KH, Johst S, Dammann P, Özkan N, Mönninghoff C, Kraemer M, et al. Improved cerebral time-of-flight magnetic resonance angiography at 7 Tesla – Feasibility study and preliminary results using optimized venous saturation pulses. PLoS One. 2014; 9:e106697. PMID:
25232868.

25. de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004; 51:22–26. PMID:
14705041.

26. Lin FH, Kwong KK, Belliveau JW, Wald LL. Parallel imaging reconstruction using automatic regularization. Magn Reson Med. 2004; 51:559–567. PMID:
15004798.

27. Michaely HJ, Herrmann KA, Kramer H, Dietrich O, Laub G, Reiser MF, et al. High-resolution renal MRA: comparison of image quality and vessel depiction with different parallel imaging acceleration factors. J Magn Reson Imaging. 2006; 24:95–100. PMID:
16729261.

28. Forman C, Piccini D, Grimm R, Hutter J, Hornegger J, Zenge MO. Reduction of respiratory motion artifacts for free-breathing whole-heart coronary MRA by weighted iterative reconstruction. Magn Reson Med. 2015; 73:1885–1895. PMID:
24912763.









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download