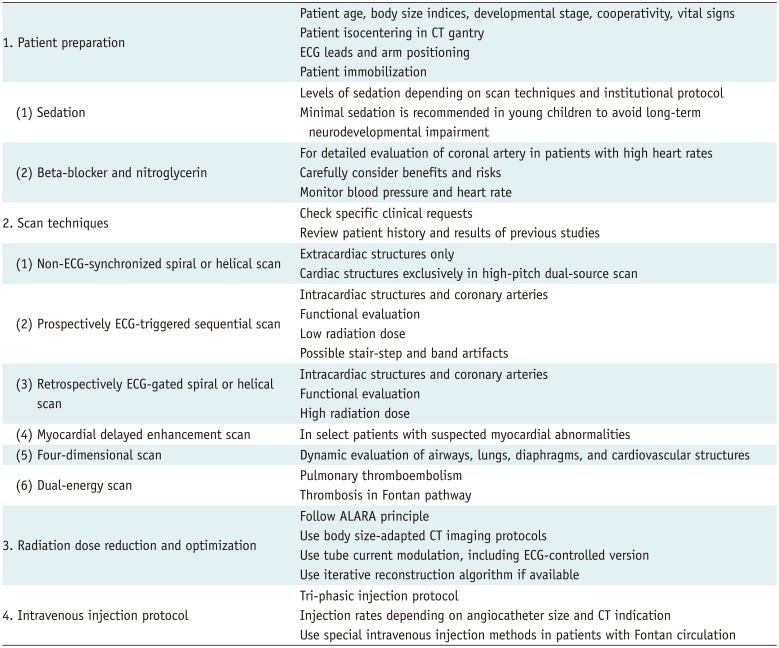
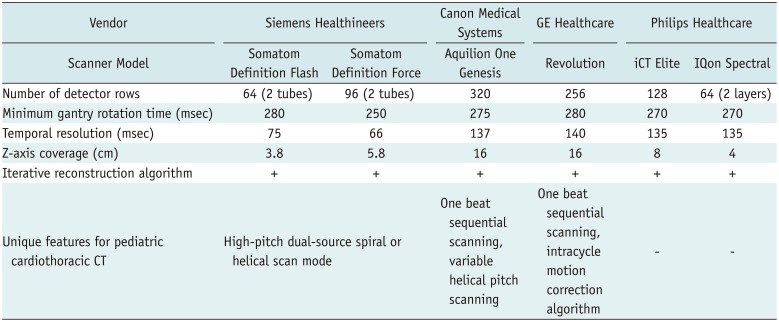
1. Han BK, Lesser AM, Vezmar M, Rosenthal K, Rutten-Ramos S, Lindberg J, et al. Cardiovascular imaging trends in congenital heart disease: a single center experience. J Cardiovasc Comput Tomogr. 2013; 7:361–366. PMID:
24331931.

2. Yang JC, Lin MT, Jaw FS, Chen SJ, Wang JK, Shih TT, et al. Trends in the utilization of computed tomography and cardiac catheterization among children with congenital heart disease. J Formos Med Assoc. 2015; 114:1061–1068. PMID:
25241602.

3. Lee T, Tsai IC, Fu YC, Jan SL, Wang CC, Chang Y, et al. Using multidetector-row CT in neonates with complex congenital heart disease to replace diagnostic cardiac catheterization for anatomical investigation: initial experiences in technical and clinical feasibility. Pediatr Radiol. 2006; 36:1273–1282. PMID:
17036235.

4. Goo HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010; 11:4–18. PMID:
20046490.

5. Goo HW. Cardiac MDCT in children: CT technology overview and interpretation. Radiol Clin North Am. 2011; 49:997–1010. PMID:
21889018.

6. Goo HW. Current trends in cardiac CT in children. Acta Radiol. 2013; 54:1055–1062. PMID:
23104372.

7. Tsai IC, Chen MC, Jan SL, Wang CC, Fu YC, Lin PC, et al. Neonatal cardiac multidetector row CT: why and how we do it. Pediatr Radiol. 2008; 38:438–451. PMID:
18259739.

8. Han BK, Rigsby CK, Hlavacek A, Leipsic J, Nicol ED, Siegel MJ, et al. Computed tomography imaging in patients with congenital heart disease Part I: rationale and utility. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr. 2015; 9:475–492. PMID:
26272851.
9. Han BK, Rigsby CK, Leipsic J, Bardo D, Abbara S, Ghoshhajra B, et al. Computed tomography imaging in patients with congenital heart disease, Part 2: technical recommendations. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr. 2015; 9:493–513. PMID:
26679548.
10. Tsai IC, Goo HW. Cardiac CT and MRI for congenital heart disease in Asian countries: recent trends in publication based on a scientific database. Int J Cardiovasc Imaging. 2013; 29(Suppl 1):1–5. PMID:
23344910.

11. Booij R, Dijkshoorn ML, van Straten M, du Plessis FA, Budde RP, Moelker A, et al. Cardiovascular imaging in pediatric patients using dual source CT. J Cardiovasc Comput Tomogr. 2016; 10:13–21. PMID:
26524989.

12. Kaasalainen T, Palmu K, Reijonen V, Kortesniemi M. Effect of patient centering on patient dose and image noise in chest CT. AJR Am J Roentgenol. 2014; 203:123–130. PMID:
24951205.

13. Goo HW. Is it better to enter a volume CT dose index value before or after scan range adjustment for radiation dose optimization of pediatric cardiothoracic CT with tube current modulation? Korean J Radiol. 2018; 19:692–703. PMID:
29962875.

14. Goo HW. Image quality and radiation dose of high-pitch dual-source spiral cardiothoracic computed tomography in young children with congenital heart disease: comparison of non-electrocardiography synchronization and prospective electrocardiography triggering. Korean J Radiol. 2018; 19:1031–1041. PMID:
30386135.

15. Tsai IC, Lee T, Chen MC, Fu YC, Jan SL, Wang CC, et al. Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr Radiol. 2007; 37:818–825. PMID:
17562037.

16. Kuo F, Plaza M, Saigal G. Inappropriate arm positioning during scout image acquisition resulting in increased radiation dose while performing a chest CT. Pediatr Radiol. 2012; 42:508–509. PMID:
22322628.

17. Diaz LK, Jones L. Sedating the child with congenital heart disease. Anesthesiol Clin. 2009; 27:301–319. PMID:
19703678.

18. Char D, Ramamoorthy C, Wise-Faberowski L. Cognitive dysfunction in children with heart disease: the role of anesthesia and sedation. Congenit Heart Dis. 2016; 11:221–229. PMID:
27228360.

19. Mahabadi AA, Achenbach S, Burgstahler C, Dill T, Fischbach R, Knez A, et al. Working group "Cardiac CT" of the German Cardiac Society. Safety, efficacy, and indications of beta-adrenergic receptor blockade to reduce heart rate prior to coronary CT angiography. Radiology. 2010; 257:614–623. PMID:
21084413.
20. Decramer I, Vanhoenacker PK, Sarno G, Van Hoe L, Bladt O, Wijns W, et al. Effects of sublingual nitroglycerin on coronary lumen diameter and number of visualized septal branches on 64-MDCT angiography. AJR Am J Roentgenol. 2008; 190:219–225. PMID:
18094315.

21. Goo HW. Coronary artery imaging in children. Korean J Radiol. 2015; 16:239–250. PMID:
25741188.

22. Kim JW, Goo HW. Coronary artery abnormalities in Kawasaki disease: comparison between CT and MR coronary angiography. Acta Radiol. 2013; 54:156–163. PMID:
23482350.

23. Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Yun TJ, et al. Visibility of the origin and proximal course of coronary arteries on non-ECG-gated heart CT in patients with congenital heart disease. Pediatr Radiol. 2005; 35:792–798. PMID:
15886981.

24. Goo HW, Seo DM, Yun TJ, Park JJ, Park IS, Ko JK, et al. Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: multislice CT findings. Pediatr Radiol. 2009; 39:265–273. PMID:
19159923.

25. Ben Saad M, Rohnean A, Sigal-Cinqualbre A, Adler G, Paul JF. Evaluation of image quality and radiation dose of thoracic and coronary dual-source CT in 110 infants with congenital heart disease. Pediatr Radiol. 2009; 39:668–676. PMID:
19319514.

26. Goo HW, Yang DH. Coronary artery visibility in free-breathing young children with congenital heart disease on cardiac 64-slice CT: dual-source ECG-triggered sequential scan vs. single-source non-ECG-synchronized spiral scan. Pediatr Radiol. 2010; 40:1670–1680. PMID:
20464385.

27. Goo HW. Identification of coronary artery anatomy on dual-source cardiac computed tomography before arterial switch operation in newborns and young infants: comparison with transthoracic echocardiography. Pediatr Radiol. 2018; 48:176–185. PMID:
29032431.

28. Rigsby CK, deFreitas RA, Nicholas AC, Leidecker C, Johanek AJ, Anley P, et al. Safety and efficacy of a drug regimen to control heart rate during 64-slice ECG-gated coronary CTA in children. Pediatr Radiol. 2010; 40:1880–1889. PMID:
20499055.

29. Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Yun TJ, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003; 23 Spec No:S147–S165. PMID:
14557509.

30. Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Park JJ. Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients. Int J Cardiovasc Imaging. 2005; 21:347–365. discussion 367. PMID:
16015453.

31. Flohr TG, Leng S, Yu L, Aiimendinger T, Bruder H, Petersilka M, et al. Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: image reconstruction and assessment of image quality. Med Phys. 2009; 36:5641–5653. PMID:
20095277.

32. Sriharan M, Lazoura O, Pavitt CW, Castellano I, Owens CM, Rubens MB, et al. Evaluation of high-pitch ungated pediatric cardiovascular computed tomography for the assessment of cardiac structures in neonates. J Thorac Imaging. 2016; 31:177–182. PMID:
27007667.

33. Kanie Y, Sato S, Tada A, Kanazawa S. Image quality of coronary arteries on non-electrocardiography-gated high-pitch dual-source computed tomography in children with congenital heart disease. Pediatr Cardiol. 2017; 38:1393–1399. PMID:
28689328.

34. Ruzsics B, Gebregziabher M, Lee H, Brothers RL, Allmendinger T, Vogt S, et al. Coronary CT angiography: automatic cardiac-phase selection for image reconstruction. Eur Radiol. 2009; 19:1906–1913. PMID:
19277670.

35. Nakanishi T, Ito K, Imazu M, Yamakido M. Evaluation of coronary artery stenoses using electron-beam CT and multiplanar reformation. J Comput Assist Tomogr. 1997; 21:121–127. PMID:
9022783.

36. Choi BW, Park YH, Choi JY, Choi BI, Kim MJ, Ryu SJ, et al. Using electron beam CT to evaluate conotruncal anomalies in pediatric and adult patients. AJR Am J Roentgenol. 2001; 177:1045–1049. PMID:
11641166.

37. Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000; 102:2823–2828. PMID:
11104739.

38. Jin KN, Park EA, Shin CI, Lee W, Chung JW, Park JH. Retrospective versus prospective ECG-gated dual-source CT in pediatric patients with congenital heart diseases: comparison of image quality and radiation dose. Int J Cardiovasc Imaging. 2010; 26(Suppl 1):63–73. PMID:
20044793.

39. Hsieh J, Londt J, Vass M, Li J, Tang X, Okerlund D. Step-and-shoot data acquisition and reconstruction for cardiac x-ray computed tomography. Med Phys. 2006; 33:4236–4248. PMID:
17153402.

40. Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008; 29:191–197. PMID:
18089704.

41. Al-Mousily F, Shifrin RY, Fricker FJ, Feranec N, Quinn NS, Chandran A. Use of 320-detector computed tomographic angiography for infants and young children with congenital heart disease. Pediatr Cardiol. 2011; 32:426–432. PMID:
21210093.

42. Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, et al. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging. 2012; 5:484–493. PMID:
22595156.
43. Labounty TM, Leipsic J, Min JK, Heilbron B, Mancini GB, Lin FY, et al. Effect of padding duration on radiation dose and image interpretation in prospectively ECG-triggered coronary CT angiography. AJR Am J Roentgenol. 2010; 194:933–937. PMID:
20308494.

44. Feuchtner G, Goetti R, Plass A, Baumueller S, Stolzmann P, Scheffel H, et al. Dual-step prospective ECG-triggered 128-slice dual-source CT for evaluation of coronary arteries and cardiac function without heart rate control: a technical note. Eur Radiol. 2010; 20:2092–2099. PMID:
20407896.

45. Goo HW. Serial changes in anatomy and ventricular function on dual-source cardiac computed tomography after the Norwood procedure for hypoplastic left heart syndrome. Pediatr Radiol. 2017; 47:1776–1786. PMID:
28879411.

46. Koyama Y, Matsuoka H, Mochizuki T, Higashino H, Kawakami H, Nakata S, et al. Assessment of reperfused acute myocardial infarction with two-phase contrast-enhanced helical CT: prediction of left ventricular function and wall thickness. Radiology. 2005; 235:804–811. PMID:
15833978.

47. Goo HW. Myocardial delayed-enhancement CT: initial experience in children and young adults. Pediatr Radiol. 2017; 47:1452–1462. PMID:
28534155.

48. Greenberg SB. Dynamic pulmonary CT of children. AJR Am J Roentgenol. 2012; 199:435–440. PMID:
22826409.

49. Goo HW. Advanced functional thoracic imaging in children: from basic concepts to clinical applications. Pediatr Radiol. 2013; 43:262–268. PMID:
23417252.

50. Goo HW, Drubach L, Lee EY. Imaging techniques. In : Coley BD, editor. Caffey's pediatric diagnostic imaging. 12th ed. Philadelphia, PA: Elsevier;2013. p. 506–518.
51. Tan JZ, Crossett M, Ditchfield M. Dynamic volumetric computed tomographic assessment of the young paediatric airway: initial experience of rapid, non-invasive, four-dimensional technique. J Med Imaging Radiat Oncol. 2013; 57:141–148. PMID:
23551770.

52. Greenberg SB, Dyamenahalli U. Dynamic pulmonary computed tomography angiography: a new standard for evaluation of combined airway and vascular abnormalities in infants. Int J Cardiovasc Imaging. 2014; 30:407–414. PMID:
24322888.

53. Goo HW. Four-dimensional CT of the diaphragm in children: initial experience. Korean J Radiol. 2018; 19:111–118. PMID:
29354007.

54. Goo HW, Goo JM. Dual-energy CT: new horizon in medical imaging. Korean J Radiol. 2017; 18:555–569. PMID:
28670151.

55. Goo HW. Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr Radiol. 2010; 40:1536–1544. PMID:
20596701.

56. Goo HW. Dual-energy lung perfusion and ventilation CT in children. Pediatr Radiol. 2013; 43:298–307. PMID:
23417255.

57. Grewal J, Al Hussein M, Feldstein J, Kiess M, Ellis J, Human D, et al. Evaluation of silent thrombus after the Fontan operation. Congenit Heart Dis. 2013; 8:40–47. PMID:
22897869.

58. Goo HW. CT radiation dose optimization and estimation: an update for radiologists. Korean J Radiol. 2012; 13:1–11. PMID:
22247630.

59. Goo HW. Individualized volume CT dose index determined by cross-sectional area and mean density of the body to achieve uniform image noise of contrast-enhanced pediatric chest CT obtained at variable kV levels and with combined tube current modulation. Pediatr Radiol. 2011; 41:839–847. PMID:
21656275.

60. Kleinman PL, Strauss KJ, Zurakowski D, Buckley KS, Taylor GA. Patient size measured on CT images as a function of age at a tertiary care children's hospital. AJR Am J Roentgenol. 2010; 194:1611–1619. PMID:
20489103.

61. Goo HW, Suh DS. Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol. 2006; 36:344–351. PMID:
16501970.

62. Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, et al. Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics. 2008; 28:1451–1459. PMID:
18794318.

63. Goo HW, Suh DS. The influences of tube voltage and scan direction on combined tube current modulation: a phantom study. Pediatr Radiol. 2006; 36:833–840. PMID:
16642311.

64. Israel GM, Herlihy S, Rubinowitz AN, Cornfeld D, Brink J. Does a combination of dose modulation with fast gantry rotation time limit CT image quality? AJR Am J Roentgenol. 2008; 191:140–144. PMID:
18562737.

65. Kordolaimi SD, Argentos S, Pantos I, Kelekis NL, Efstathopoulos EP. A new era in computed tomographic dose optimization: the impact of iterative reconstruction on image quality and radiation dose. J Comput Assist Tomogr. 2013; 37:924–931. PMID:
24270114.
66. Han BK, Grant KL, Garberich R, Sedlmair M, Lindberg J, Lesser JR. Assessment of an iterative reconstruction algorithm (SAFIRE) on image quality in pediatric cardiac CT datasets. J Cardiovasc Comput Tomogr. 2012; 6:200–204. PMID:
22682262.

67. Rompel O, Glöckler M, Janka R, Dittrich S, Cesnjevar R, Lell MM, et al. Third-generation dual-source 70-kVp chest CT angiography with advanced iterative reconstruction in young children: image quality and radiation dose reduction. Pediatr Radiol. 2016; 46:462–472. PMID:
26739141.

68. Shirota G, Maeda E, Namiki Y, Bari R, Ino K, Torigoe R, et al. Pediatric 320-row cardiac computed tomography using electrocardiogram-gated model-based full iterative reconstruction. Pediatr Radiol. 2017; 47:1463–1470. PMID:
28667349.

69. Infante JC, Liu Y, Rigsby CK. CT image quality in sinogram affirmed iterative reconstruction phantom study - is there a point of diminishing returns? Pediatr Radiol. 2017; 47:333–341. PMID:
27891546.

70. Lee KB, Goo HW. Quantitative image quality and histogram-based evaluations of an iterative reconstruction algorithm at low-to-ultralow radiation dose levels: a phantom study in chest CT. Korean J Radiol. 2018; 19:119–129. PMID:
29354008.

71. Deak PD, Langner O, Lell M, Kalender WA. Effects of adaptive section collimation on patient radiation dose in multisection spiral CT. Radiology. 2009; 252:140–147. PMID:
19561253.

72. Kim YK, Sung YM, Choi JH, Kim EY, Kim HS. Reduced radiation exposure of the female breast during low-dose chest CT using organ-based tube current modulation and a bismuth shield: comparison of image quality and radiation dose. AJR Am J Roentgenol. 2013; 200:537–544. PMID:
23436842.

73. Taylor S, Litmanovich DE, Shahrzad M, Bankier AA, Gevenois PA, Tack D. Organ-based tube current modulation: are women’s breasts positioned in the reduced-dose zone? Radiology. 2015; 274:260–266. PMID:
25153159.

74. Lee E, Goo HW, Lee JY. Age- and gender-specific estimates of cumulative CT dose over 5 years using real radiation dose tracking data in children. Pediatr Radiol. 2015; 45:1282–1292. PMID:
25801905.
75. Hui PKT, Goo HW, Du J, Ip JJK, Kanzaki S, Kim YJ, et al. Asian consortium on radiation dose of pediatric cardiac CT (ASCI-REDCARD). Pediatr Radiol. 2017; 47:899–910. PMID:
28435986.

76. Litmanovich D, Zamboni GA, Hauser TH, Lin PJ, Clouse ME, Raptopoulos V. ECG-gated chest CT angiography with 64-MDCT and tri-phasic IV contrast administration regimen in patients with acute non-specific chest pain. Eur Radiol. 2008; 18:308–317. PMID:
17763855.

77. Goo HW. Haemodynamic findings on cardiac CT in children with congenital heart disease. Pediatr Radiol. 2011; 41:250–261. PMID:
21127855.

78. Ghadimi Mahani M, Agarwal PP, Rigsby CK, Lu JC, Fazeli Dehkordy S, Wright RA, et al. CT for assessment of thrombosis and pulmonary embolism in multiple stages of single-ventricle palliation: challenges and suggested protocols. Radiographics. 2016; 36:1273–1284. PMID:
27618316.

79. Greenberg SB, Bhutta ST. A dual contrast injection technique for multidetector computed tomography angiography of Fontan procedures. Int J Cardiovasc Imaging. 2008; 24:345–348. PMID:
17823851.

80. Prabhu SP, Mahmood S, Sena L, Lee EY. MDCT evaluation of pulmonary embolism in children and young adults following a lateral tunnel Fontan procedure: optimizing contrast-enhancement techniques. Pediatr Radiol. 2009; 39:938–944. PMID:
19471914.

81. Sandler KL, Markham LW, Mah ML, Byrum EP, Williams JR. Optimizing CT angiography in patients with Fontan physiology: single-center experience of dual-site power injection. Clin Radiol. 2014; 69:e562–e567. PMID:
25446326.

82. Park EA, Lee W, Chung SY, Yin YH, Chung JW, Park JH. Optimal scan timing and intravenous route for contrast-enhanced computed tomography in patients after Fontan operation. J Comput Assist Tomogr. 2010; 34:75–81. PMID:
20118726.

83. Johnson PT, Christensen GM, Fishman EK. I.v. contrast administration with dual source 128-MDCT: a randomized controlled study comparing 18-gauge nonfenestrated and 20-gauge fenestrated catheters for catheter placement success, infusion rate, image quality, and complications. AJR Am J Roentgenol. 2014; 202:1166–1170. PMID:
24848812.

84. Tamura A, Kato K, Kamata M, Suzuki T, Suzuki M, Nakayama M, et al. Selection of peripheral intravenous catheters with 24-gauge side-holes versus those with 22-gauge end-hole for MDCT: a prospective randomized study. Eur J Radiol. 2017; 87:8–12. PMID:
28065379.

85. Choo KS, Lee HD, Ban JE, Sung SC, Chang YH, Kim CW, et al. Evaluation of obstructive airway lesions in complex congenital heart disease using composite volume-rendered images from multislice CT. Pediatr Radiol. 2006; 36:219–223. PMID:
16391927.

86. Hong SH, Kim YM, Lee HJ. Three-dimensional endo-cardiovascular volume-rendered cine computed tomography of isolated left ventricular apical hypoplasia: a case report and literature review. Korean J Radiol. 2016; 17:79–82. PMID:
26798219.

87. Kim HJ, Goo HW, Park SH, Yun TJ. Left ventricle volume measured by cardiac CT in an infant with a small left ventricle: a new and accurate method in determining uni- or biventricular repair. Pediatr Radiol. 2013; 43:243–246. PMID:
22875206.

88. Goo HW, Park SH. Semiautomatic three-dimensional CT ventricular volumetry in patients with congenital heart disease: agreement between two methods with different user interaction. Int J Cardiovasc Imaging. 2015; 31(Suppl 2):223–232. PMID:
26319216.

89. Goo HW, Park SH. Pulmonary vascular volume ratio measured by cardiac computed tomography in children and young adults with congenital heart disease: comparison with lung perfusion scintigraphy. Pediatr Radiol. 2017; 47:1580–1587. PMID:
28646273.

90. Goo HW. Computed tomography pulmonary vascular volume ratio in children and young adults with congenital heart disease: the effect of cardiac phase. Pediatr Radiol. 2018; 48:915–922. PMID:
29572746.

91. Goo HW. Free-breathing cine CT for the diagnosis of tracheomalacia in young children. Pediatr Radiol. 2013; 43:922–928. PMID:
23417231.

92. Goo HW, Kim HJ. Detection of air trapping on inspiratory and expiratory phase images obtained by 0.3-second cine CT in the lungs of free-breathing young children. AJR Am J Roentgenol. 2006; 187:1019–1023. PMID:
16985151.

93. Yoo SJ, Spray T, Austin EH 3rd, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017; 153:1530–1540. PMID:
28268011.

94. Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006; 16:256–268. PMID:
16341833.

95. Bodelle B, Fischbach C, Booz C, Yel I, Frellesen C, Beeres M, et al. Free-breathing high-pitch 80kVp dual-source computed tomography of the pediatric chest: image quality, presence of motion artifacts and radiation dose. Eur J Radiol. 2017; 89:208–214. PMID:
28267541.
96. Lell MM, May M, Deak P, Alibek S, Kuefner M, Kuettner A, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011; 46:116–123. PMID:
20856124.
97. Nie P, Wang X, Cheng Z, Ji X, Duan Y, Chen J. Accuracy, image quality and radiation dose comparison of high-pitch spiral and sequential acquisition on 128-slice dual-source CT angiography in children with congenital heart disease. Eur Radiol. 2012; 22:2057–2066. PMID:
22592808.

98. Zheng M, Zhao H, Xu J, Wu Y, Li J. Image quality of ultra-low-dose dual-source CT angiography using high-pitch spiral acquisition and iterative reconstruction in young children with congenital heart disease. J Cardiovasc Comput Tomogr. 2013; 7:376–382. PMID:
24331933.

99. Xu J, Zhao H, Wang X, Bai Y, Liu L, Liu Y, et al. Accuracy, image quality, and radiation dose of prospectively ECG-triggered high-pitch dual-source CT angiography in infants and children with complex coarctation of the aorta. Acad Radiol. 2014; 21:1248–1254. PMID:
25097011.

100. Goo HW. Comparison of chest pain protocols for electrocardiography-gated dual-source cardiothoracic CT in children and adults: the effect of tube current saturation on radiation dose reduction. Korean J Radiol. 2018; 19:23–31. PMID:
29353996.

101. Nakagawa M, Ozawa Y, Sakurai K, Shimohira M, Ohashi K, Asano M, et al. Image quality at low tube voltage (70 kV) and sinogram-affirmed iterative reconstruction for computed tomography in infants with congenital heart disease. Pediatr Radiol. 2015; 45:1472–1479. PMID:
26115723.

102. Niemann T, Henry S, Faivre JB, Yasunaga K, Bendaoud S, Simeone A, et al. Clinical evaluation of automatic tube voltage selection in chest CT angiography. Eur Radiol. 2013; 23:2643–2651. PMID:
23828227.

103. Matsumoto S, Yamada Y, Hashimoto M, Okamura T, Yamada M, Yashima F, et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol. 2017; 27:1963–1970. PMID:
27562479.

104. Leipsic J, Labounty TM, Hague CJ, Mancini GB, O'Brien JM, Wood DA, et al. Effect of a novel vendor-specific motion-correction algorithm on image quality and diagnostic accuracy in persons undergoing coronary CT angiography without rate-control medications. J Cardiovasc Comput Tomogr. 2012; 6:164–171. PMID:
22551593.

105. Rajiah P, Abbara S, Halliburton SS. Spectral detector CT for cardiovascular applications. Diagn Interv Radiol. 2017; 23:187–193. PMID:
28302592.

106. Jia Q, Zhuang J, Jiang J, Li J, Huang M, Liang C. Image quality of CT angiography using model-based iterative reconstruction in infants with congenital heart disease: comparison with filtered back projection and hybrid iterative reconstruction. Eur J Radiol. 2017; 86:190–197. PMID:
28027746.

107. Lewis MA, Pascoal A, Keevil SF, Lewis CA. Selecting a CT scanner for cardiac imaging: the heart of the matter. Br J Radiol. 2016; 89:20160376. PMID:
27302494.