Abstract
Background
Although Th2 immune activation is predominant in allergic diseases, neopterinlevels and indoleamine 2,3-dioxygenase (IDO)-1 activity (kynurenine:tryptophan ratio), which reflect Th1 immune activity, increase with interferon-gamma (IFN-γ) stimulation. We investigated neopterin, tryptophan, and kynurenine levels as biomarkersof the Th1 immune system activation and changes in IDO-1 activityin children with asthma, allergic rhinitis, and atopic dermatitis, as well as the relationship between these biomarkers and the total IgE level, age, and disease severity.
Methods
We divided 205 children (80 girls and 125 boys, four months to 17 years old) into four groups: controls, patients with asthma, patients with allergic rhinitis, and patients with atopic dermatitis. Peripheral venous blood samples were collected. Neopterin levels were determined by an enzyme immunoassay. Tryptophan and kynurenine levels were analyzed using HPLC. IDO-1 enzyme activity was calculated using tryptophan and kynurenine levels. IgE levels were measured. The Mann-Whitney U test, Kruskal-Wallis test, and Conover post-hoc method were used for statistical analysis.
Results
Neopterin, tryptophan, and kynurenine levels were higher and IgE levels and IDO-1 enzyme activity were lower in patients with asthma and allergic rhinitis than in controls (P<0.05). Patients with atopic dermatitis showed higher neopterin, tryptophan, and kynurenine levels, higher IDO-1 activity, and lower IgE levels thancontrols (P<0.05).
Allergic diseases, one of the most common chronic conditions in childhood, include allergic rhinitis, asthma, dermatitis, conjunctivitis, anaphylaxis, and food and drug allergies, and affect approximately one in five people globally [12]. Allergies are immune reactions that affect various tissues and organs in many diseases [3]. Asthma, allergic rhinitis, and atopic dermatitis, also referred to as atopic diseases, develop from a complex genetic background, known as atopy [45]. The prevalence of these three atopic diseases increases gradually. Although the diseases in this group occur in different organs, increased total serum IgE levels are observed in most patients [5].
In patients with atopic dermatitis, cells expressing Th2 cytokines are increased whereas cells expressing Th1 cytokines are often decreased in the peripheral blood [6]. Langerhans cells present in the epidermis of patients with allergic dermatitis express receptors with a high affinity for IgE, and their expression is associated with the severity of allergic dermatitis, asthma, and allergic rhinitis [37]. Allergic rhinitis poses a risk factor in asthma since under certain conditions it leads to asthma [3], and under uncontrolled moderate and severe conditions, it affects asthma control [8]. The prevalence of asthma increases gradually in Europe (8.2% in adults and 9.4% in children) [9]. It varies worldwide, depending on race, geographic region, and environmental factors [10].
The cell-mediated immune response typically depends on the activation of Th1 cells, which produce and release IFN-γ and interleukin (IL)-2, whereas activation of Th2 cells is characterized by the release of cytokines such as IL-4, IL-5, and IL-10. Th2 cytokines are responsible for the humoral immune response and trigger the production of immunoglobulins, such as IgE, which are involved in allergic diseases. The cross-regulatory interaction between Th1 and Th2 immune responses is reflected in IgE and neopterin levels [11]. Tryptophan catabolism is firmly established as a key regulator of innate and adaptive immune tolerance [1213]. The tryptophan to kynurenine metabolic pathway is catalyzed mainly by three enzymes: indoleamine 2,3-dioxygenase (IDO)-1, IDO-2, and tryptophan 2,3-dioxygenase (TDO) [1415]. IDO-1 catalyzes the rate-limiting step in tryptophan degradation in the kynurenine pathway [16]. While IDO-1 is widely expressed in various cells and tissues, TDO is expressed mainly in the hepatic tissue and is thought to be the main enzyme converting tryptophan to kynurenine in the liver. Thus, the serum kynurenine: tryptophan ratio reflects IDO-1activity [1215].
Th1- and Th2-mediated immune responses downregulate each other. Th1 cells stimulate human monocytes/macrophages by producing IFN-γ and IL-2, which results in the production and release of large amounts of neopterin, which in turn downregulates the humoral IgE-mediated immune response [17]. Hence, we evaluated the cross-regulatory interaction between Th1- and Th2-mediated immune responses in various important allergic diseases in children. To gain insight into this interaction, the cellular immune system activation biomarkers were measured and compared with the serum IgE levels, one of the Th2 cytokines. We investigated the association of Th1 immunity indicators, including neopterin levels, tryptophan breakdown, and IDO-1 activity as biomarkers in children with allergic diseases. Further, we evaluated changes in the studied biomarkers according to age and gender, the relationship between these biomarkers and disease severity, and the effects of these biomarkers on allergic and non-allergic forms. In addition, we compared Th2 cytokine IgE levels with Th1 immunity indicators.
This prospective study, conducted from February to August 2014, included 205 children in total: 183 children aged between four months and 17 years (median: six years) who were admitted to the Division of Pediatric Allergy and Immunology, Department of Pediatrics, Inonu University, Faculty of Medicine, Malatya (Turkey), and who were diagnosed with asthma, allergic rhinitis, oratopic dermatitis, and 22 healthy children as controls (median: eight years; range: 3–16 years). The study was approved by the Ethics Committee of İnönü University (document number: 2013/32), and all parents provided written informed consent. Demographic characteristics of the study population are presented in Table 1.
Patients with medical conditions that may cause Th1 activation (e.g., cancer, autoimmune disease, infections, diabetes, heart failure, kidney failure, and chronic hepatitis) were excluded. Both patients, who received medication and those who did not receive treatment, were included. Some patients received inhaled steroid treatment, and montelukast treatment was applied when appropriate. An asthma attack was defined as an episode of progressive increase in shortness of breath, cough, wheezing, or chest tightness, or a combination of some or all of these symptoms. The diagnosis, severity, and control level of asthma were assessed according to the guidelines of the Global Initiative for Asthma (GINA) [18]. According to the severity of asthma, it was classified as intermittent, mild persistent, moderate persistent, and severe persistent. The patients were divided into three subgroups according to the level of asthma: controlled, partly controlled, and uncontrolled asthma. Allergic rhinitis was diagnosed and classified (as seasonal or perennial, and mild, moderate, or severe, and allergic or non-allergic) according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines [14]. Atopic dermatitis was diagnosed according to the Hanifin and Rajka criteria based on patient history and clinical characteristics [19]. Subgroups of asthma and atopic dermatitis patients are presented in Table 2.
Peripheral venous blood samples (1–2 mL) were collected early in the morning from all patients and controls. The samples were centrifuged at 3,220 ×g for 15 minutes at 20–25℃, after which the sera were separated to measure neopterin, tryptophan, and kynurenine levels. All samples were stored at −20℃ until analysis. Serum neopterin levels were determined by an enzyme immunoassay using a commercially available neopterin assay (Human Serum Neopterin Assay, EIA-1476, DRG Instruments GmbH, Marburg, Germany) as per the manufacturer's instructions. Absorbance was read at 450 nm in a microplate reader. Serum neopterin levels were expressed in nM, and the lowest detectable neopterin level was 0.8 nM. Serum tryptophan and kynurenine levels were measured by the HP Agilent 1100 HPLC system (Agilent, Vienna, Austria) using a reversed-phase Hichrom C18 column (5 µm, 25 cm×4.6 mm i.d., Hichrom Ltd., Berkshire, UK). The proteins were precipitated with perchloric acid. Tryptophan level was determined through natural fluorescence detection (G1312A; Agilent) at 285 nm excitation and 360 nm emission wavelengths, whereas kynurenine level was determined through variable wavelength detection (G1314A; Agilent) with 360-nm wavelength ultraviolet (UV) absorption [2021]. Both tryptophan and kynurenine levels were expressed in µM/L. IDO-1 enzyme activity was evaluated on the basis of serum tryptophan and kynurenine levels and the kynurenine: tryptophan ratio. IDO-1 activity was expressed in µM/mM.
Categorical variables were summarized as frequencies and percentages, whereas non-normally distributed continuous variables were summarized as median and range. The Mann–Whitney U-test was used to compare two independent groups, and the Kruskal–Wallis test and Conover post-hoc method were used to compare more than two groups. Spearman's rank correlation coefficient was used to evaluate relationships between variables. All analyses were performed using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA), and P<0.05 was considered statistically significant.
IDO-1 was higher in the patients with acute asthma exacerbation than in the patients who were not suffering an acute asthma attack (57.06 vs 46.24 µM/mM; P=0.028). Tryptophan levels were higher in the patients with controlled asthma (61.60 µM/L) than in the patients with partially controlled (55.98 µM/L) and uncontrolled (49.40 µM/L) asthma (P=0.025).
Patients with atopic dermatitis were divided into two subgroups as acute exacerbation positive and acute exacerbation negative. Kynurenine levels were higher in the patients with acute exacerbation of atopic dermatitis than in those who did not have acute exacerbation (3.58 vs 2.56 µM/L; P=0.005). Results forIDO-1 activity were similar to those obtained for kynurenine levels. IDO-1 activity was higher in the patients with acute exacerbation of atopic dermatitis than in the patients who did not have acute exacerbation (64.80 vs 45.26 µM/L; P=0.026). The percentage of skin affected by atopic dermatitis and the neopterin level were moderately correlated (r=0.680; P=0.001). Parameters did not significantly differ between the subgroups of allergic rhinitis and non-allergic rhinitis or by severity of allergic rhinitis. The biomarkers evaluated in the study groups are summarized in Table 3. The highest neopterin and kynurenine levels and the highest IDO-1 activity were observed in patients with atopic dermatitis, and the highest tryptophan levels were observed in patients with asthma. The lowest total IgE levels were observed in patients with atopic dermatitis, whereas the lowest IDO-1 activity was observed in patients with allergic rhinitis.
Correlations between biomarkers and age are presented in Table 4. Gender did not significantly correlate with any biomarker. Age was weakly correlated with neopterin level and moderately correlated with IgE level in patients with allergic rhinitis. Age was weakly correlated with IDO-1 activity in patients with asthma and allergic rhinitis and was moderately correlated in atopic dermatitis patient group.
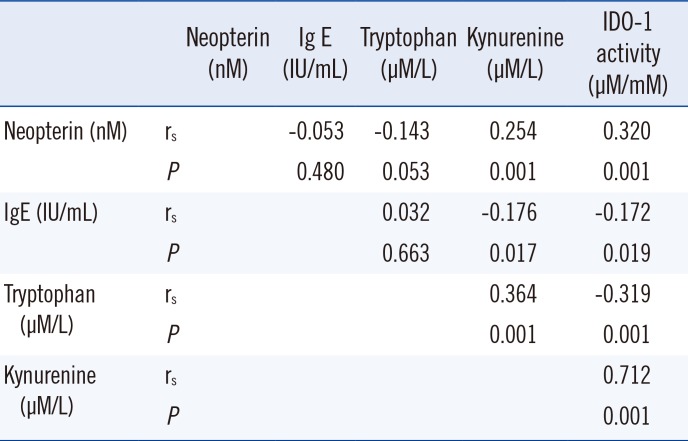
Correlations between biomarkers in the study groups are presented in Table 5. Kynurenine was very weakly correlated with IgE, neopterin,and tryptophan levels. IDO-1 activity was very weakly correlated with IgE and tryptophan levels. IDO-1 activity was weakly correlated with neopterin and was strongly correlated with kynurenine levels.
Allergic diseases, such as asthma, rhinitis, and atopic dermatitis, are characterized by Th2-skewed immunity. Th2 cytokines are upregulated in allergic inflammation, whereas the Th1 immune response and Th1-related cytokines, such as IFN-γ, are downregulated [222324]. The biochemical breakdown of tryptophan by IDO-1 is controlled by IFN-γ, which is responsible for the strongest Th1-mediated immune response against proinflammatory stimuli [22].
Neopterin is produced from guanosine triphosphate (GTP) by monocytes and macrophages that are activated by T-lymphocytes. Neopterin is found in normal quantities in the serum (4.9 nM) and urine (1.58 mM/1.2 L/day) and is removed through renal excretion (clearance; 225 mL/min) [25]. Neopterin has become key in the diagnosis and follow-up of various pathologies, as it indicates cellular immune activation and can be easily monitored in biological fluids using sensitive assays [172627].
Tryptophan, which is related to neopterin, a product of the pteridine pathway, and kynurenine, which is the main degradation product of tryptophan, are involved in various metabolic disorders, depression, autoimmune diseases, malignancies, and neurodegenerative diseases [2829]. The mechanisms involved in tryptophan degradation play a role in the regulation of immune responses in various disorders that develop in response to genetic, environmental, and immunological factors [1430]. Furthermore, increased tryptophan degradation has been reported in patients with various diseases, such as rheumatoid arthritis, viral infections, and certain malignancies that exhibit poor prognosis [3132333435].
Under normal physiological conditions, the kynurenine level is related to that of tryptophan. IDO assumes the major role, although tryptophan 2,3-dioxygenase (TDO) may also play a part in immune system activation [36].
In our study, total serum IgE levels of the patients with allergic rhinitis and atopic dermatitis were significantly lower than those of controls. This finding may be related to the lower mean age of patients with atopic dermatitis than that in the other groups. The increase in the total serum IgE level in childhood becomes more remarkable with age. The highest IgE levels were observed in patients with allergic rhinitis, who had the highest mean age. In patients with allergic rhinitis, a moderate correlation between IgE levels and age was identified. The overall age range among all diagnostic groups was large. Between five and six years, the immune system completes maturation, and the levels of various biomarkers noticeably change [37].
In allergic diseases, increased serum neopterin levels and decreased serum IgE levels can sometimes be noted due to a disruption of the Th1/Th2 balance, although some patients show decreased serum neopterin levels and increased serum IgE levels [11]. In our study, neopterin levels were higher and IgE levels lower in all the patient groups than in the control group. Similarly, Pinto et al. [17] reported higher neopterin levels in patients with allergic diseases than in the control group. Among the patient groups, the highest neopterin levels were found in atopic dermatitis patients, who had the lowest IgE levels, whereas the lowest neopterin levels were found in allergic rhinitis patients, who had the highest IgE levels, which may be caused by a disease-related disruption of the Th1/Th2 balance.
Neopterin levels and IDO activity are important indicators of a Th1 immune response. Enhanced systemic IDO activity may contribute to the containment of allergic T-cell responses and the inhibition of specific allergic reactions in allergen-sensitized individuals without clinically apparent disease [7]. In our study, similar to neopterin levels, the highest IDO-1 activity was observed in patients with atopic dermatitis, and the lowest IDO-1 activity was observed in patients with allergic rhinitis. A previous study revealed that atopy was associated with low IDO-1 activity [38]. In our study groups, IDO-1 activity was inversely correlated with IgE levels, as was the case for tryptophan levels. The genetic, humoral, and cellular differences between patients with allergic and non-allergic forms of atopic diseases reflect the presence of a complex immunological network. Local IgE production in the affected tissue is a common feature in all subtypes of atopic disease [5]. Elevated IgE usually indicates the atopy underlying allergic diseases, such as asthma, rhinoconjunctivitis, and eczema, although not all Th2-mediated immune responses are characterized by IgE production [22]. It was observed that allergic and non-allergic forms did not affect the biomarkers in patients with allergic rhinitis. All biomarkers except IDO-1 activity, i.e., tryptophan, kynurenine, and IgE levels, were higher, albeit not significantly, in allergic than in non-allergic patients.
Neopterin levels and the rate of tryptophan degradation differ in many immune system-mediated pathologies, and thus, determining the serum neopterin level and the tryptophan degradation rate in pediatric allergic conditions may contribute to the early detection of immune alterations. Ciprandi et al. [36] reported higher serum tryptophan and kynurenine levels in allergic rhinitis patients than in controls, and they suggested that IDO and neopterin unexpectedly behave oppositely. Similarly, Kositz et al. [23] reported higher tryptophan levels in atopics than in healthy blood donors, and suggested that higher tryptophan levels may result from lower IDO-1 activity in atopics. Serum tryptophan levels are higher in patients with pollen allergy than in healthy blood donors [22], possibly because of suppression of IDO-1 enzyme activity.
Our preliminary study suggests that Th1 immune activation biomarkers as an alternative to, or in combination with, IgE levels, could be used as a biomarker in conditions, in which IgE levels have limited usefulness for diagnosis and follow-up. A predominance of Th2-mediated immunity underlies the immunological basis of asthma, allergic rhinitis, and atopic dermatitis, and in parallel to this, the Th1-mediated immune response is suppressed by Th2 cytokines. Tryptophan degradation, neopterin production, and IDO-1 competence are among the biochemical pathways triggered by IFN-γ. However, the present study revealed opposite effects. Our results suggest that the Th1/Th2 balance is disrupted in patients with allergic diseases, concomitant with increased Th1-mediated immune response activation and reduced IgE production, which is promoted by Th2-type cytokines. However, decreased IgE levels alone do not categorically support the suggestion that Th2-mediated immunity is suppressed. Further studies are required to elucidate the immunological mechanisms of this complex group of disorders.
The most important limitation of this study is that the age range significantly differed among the study groups, and this is expected to have affected the biomarker values. Nevertheless, we showed that the assessment of Th1-mediated immune system parameters in childhood allergic diseases is important. The clinical use of these cheap and rapidly measurable parameters may contribute to early diagnosis and treatment of allergic diseases.
References
1. Haanpää L, af Ursin P, Nermes M, Kaljonen A, Isolauri E. Association of allergic diseases with children's life satisfaction: population-based study in Finland. BMJ Open. 2018; 8:e019281.
2. Pawankar R, Canonica GW, et al. World Allergy Organisation (WAO) white book on allergy: update 2013. Milwaukee (WI): World Allergy Organization;2013. p. 1–10.
3. Spergel JM. Atopic march: link to upper airways. Curr Opin Allergy Clin Immunol. 2005; 5:17–21. PMID: 15643339.
4. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and Aller-Gen). Allergy. 2008; 63:8–160. PMID: 18331513.
5. Novak N, Bieber T. Allergic and nonallergic forms of atopic diseases. J Allergy Clin Immunol. 2003; 112:252–262. PMID: 12897728.
6. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011; 2:110. PMID: 21994899.
7. von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin Exp Allergy. 2004; 34:1056–1063. PMID: 15248850.
8. Braido F. Failure in asthma control: reasons and consequences. Scientifica (Cairo). 2013; 2013:549252. PMID: 24455432.
9. Selroos O, Kupczyk M, Kuna P, Łacwik P, Bousquet J, Brennan D, et al. National and regional asthma programmes in Europe. Eur Respir Rev. 2015; 24:474–483. PMID: 26324809.
10. Malhotra K, Baltrus P, Zhang S, McRoy L, Immergluck LC, Rust G. Geographic and racial variation in asthma prevalence and emergency department use among Medicaid-enrolled children in 14 southern states. J Asthma. 2014; 51:913–921. PMID: 24915006.
11. Ledochowski M, Murr C, Widner B, Fuchs D. Inverse relationship between neopterin and immunoglobulin E. Clin Immunol. 2001; 98:104–108. PMID: 11141332.
12. Ciprandi G, De Amici M, Tosca M, Fuchs D. Tryptophan metabolism in allergic rhinitis: the effect of pollen allergen exposure. Hum Immunol. 2010; 71:911–915. PMID: 20540982.
13. Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2015; 5:673. PMID: 25628622.
14. Badawy AA. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int J Tryptophan Res. 2017; 10:1178646917691938. PMID: 28469468.
15. Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring). 2015; 23:2066–2074. PMID: 26347385.
16. Luukkainen A, Karjalainen J, Honkanen T, Lehtonen M, Paavonen T, Toppila-Salmi S. Indoleamine 2,3-dioxygenase expression in patients with allergic rhinitis: a case-control study. Clin Transl Allergy. 2011; 1:17. PMID: 22410120.
17. Mota Pinto A, Todo Bom A, Vale Pereira S, Alves V, Santos Rosa M. Elevated neopterin levels in non-allergic asthma. Pathophysiology. 2007; 14:35–39. PMID: 17113763.
18. Global initiative for asthma GINA Report: Global strategy for asthma management and prevention. Updated on Feb 2018. https://ginasthma.org/gina-reports/.
19. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; 92:44–47.
20. Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997; 43:2424–2426. PMID: 9439467.
21. Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 2002; 48:579–581. PMID: 11861457.
22. Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan metabolism in allergic disorders. Int Arch Allergy Immunol. 2016; 169:203–215. PMID: 27161289.
23. Kositz C, Schroecksnadel K, Grander G, Schennach H, Kofler H, Fuchs D. High serum tryptophan concentration in pollinosis patients is associated with unresponsiveness to pollen extract therapy. Int Arch Allergy Immunol. 2008; 147:35–40. PMID: 18446051.
24. Ciprandi G, Fuchs D. Tryptophan metabolic pathway, airway nitric oxide, and allergy. Ann Allergy Asthma Immunol. 2017; 119:395–396. PMID: 29150065.
25. Fuchs D, Stahl-Hennig C, Gruber A, Murr C, Hunsmann G, Wachter H. Neopterin--its clinical use in urinalysis. Kidney Int Suppl. 1994; 47:S8–S11. PMID: 7869677.
26. Eisenhut M. Neopterin in diagnosis and monitoring of infectious diseases. J Biomark. 2013; 2013:196432. PMID: 26317013.
27. Shirai R, Sato K, Yamashita T, Yamaguchi M, Okano T, Watanabe-Kominato K, et al. Neopterin counters vascular inflammation and atherosclerosis. J Am Heart Assoc. 2018; 7:e007359. PMID: 29420219.
28. Pompili M, Lionetto L, Curto M, Forte A, Erbuto D, Montebovi F, et al. Tryptophan and kynurenine metabolites: are they related to depression? Neuropsychobiology. 2019; 77:23–28. PMID: 30110684.
29. Zuo H, Ueland PM, Ulvik A, Eussen SJ, Vollset SE, Nygård O, et al. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health Study. Am J Epidemiol. 2016; 183:249–258. PMID: 26823439.
30. Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PH, et al. Tryptophan as a link between psychopathology and somatic states. Psychosom Med. 2003; 65:665–671. PMID: 12883120.
31. Kurz K, Schroecksnadel S, Weiss G, Fuchs D. Association between increased tryptophan degradation and depression in cancer patients. Curr Opin Clin Nutr Metab Care. 2011; 14:49–56. PMID: 21076293.
32. Capuron L, Geisler S, Kurz K, Leblhuber F, Sperner-Unterweger B, Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr Pharm Des. 2014; 20:6048–6057. PMID: 24641220.
33. Sucher R, Kurz K, Weiss G, Margreiter R, Fuchs D, Brandacher G. IDO-mediated tryptophan degradation in the pathogenesis of malignant tumor disease. Int J Tryptophan Res. 2010; 3:113–120. PMID: 22084593.
34. Luukkainen A, Karjalainen J, Honkanen T, Lehtonen M, Paavonen T, Toppila-Salmi S. Indoleamine 2,3-dioxygenase expression in patients with allergic rhinitis: a case-control study. Clin Transl Allergy. 2011; 1:17. PMID: 22410120.
35. Champe PC, Harvey RA. Biochemistry, Lippincott's illustrated reviews. J.B. Lippincott Publishers Company;1994. p. 457–472.
36. Ciprandi G, De Amici M, Tosca M, Fuchs D. Tryptophan metabolism in allergic rhinitis: the effect of pollen allergen exposure. Hum Immunol. 2010; 71:911–915. PMID: 20540982.
37. Girgin G, Baydar T, Fuchs D, Sahin G, Özmert E, Yurdakök K. Evaluation of serum and urinary levels of some pteridine pathway components in healthy Turkish children. Pteridines. 2013; 23:90–95.
38. Luukkainen A, Karjalainen J, Hurme M, Paavonen T, Huhtala H, Toppila-Salmi S. Relationships of indoleamine 2,3-dioxygenase activity and cofactors with asthma and nasal polyps. Am J Rhinol Allergy. 2014; 28:e5–e10. PMID: 24717869.
Table 1
Demographic data of the study population
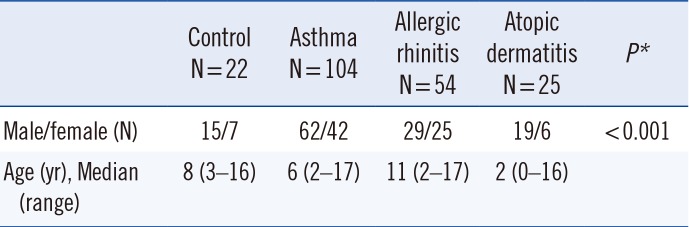
| Control N=22 | Asthma N=104 | Allergic rhinitis N=54 | Atopic dermatitis N=25 | P* | |
|---|---|---|---|---|---|
| Male/female (N) | 15/7 | 62/42 | 29/25 | 19/6 | <0.001 |
| Age (yr), Median (range) | 8 (3–16) | 6 (2–17) | 11 (2–17) | 2 (0–16) |
Table 2
Disease subgroups in asthma patients and atopic dermatitis patients
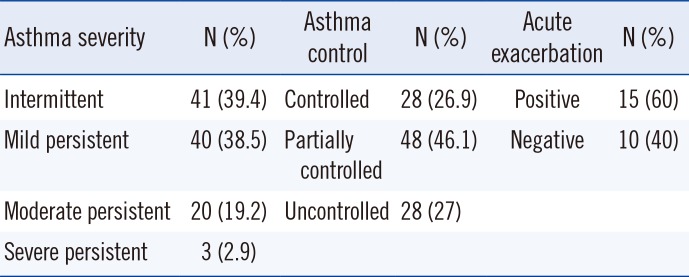
Table 3
Parameter measurements in the study groups
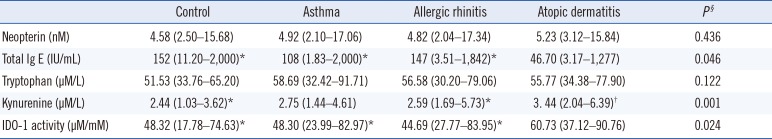
Table 4
Correlations between age and levelsof biomarkers
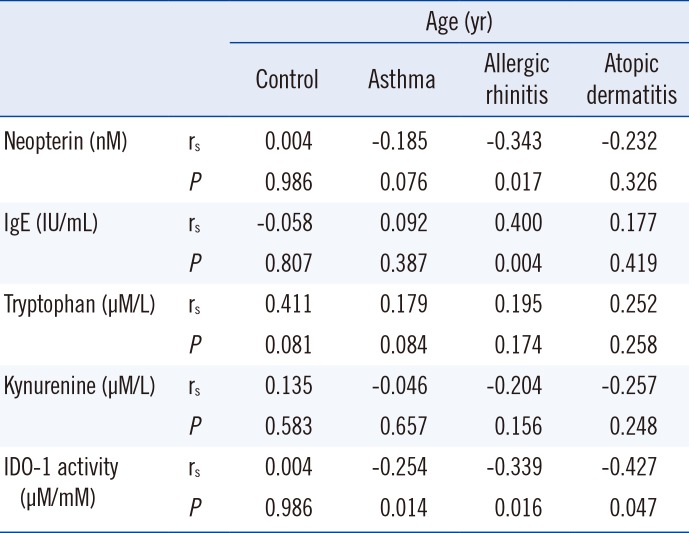
Table 5
Correlations between biomarkers in the study groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download