INTRODUCTION
METHODS
Reagents, instruments, analytical conditions, and sample preparation
Table 1
HPLC gradient conditions and mass spectrometer operating conditions
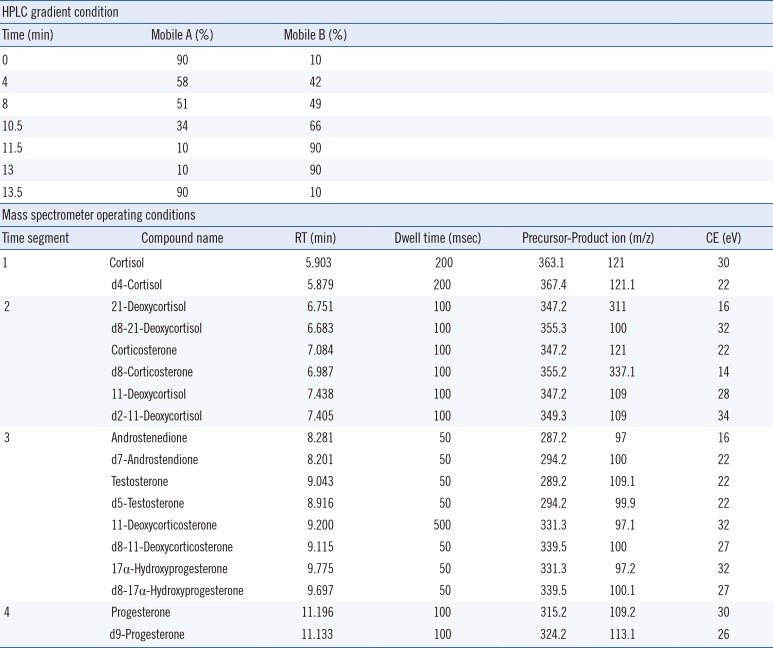
| HPLC gradient condition | ||||||
|---|---|---|---|---|---|---|
| Time (min) | Mobile A (%) | Mobile B (%) | ||||
| 0 | 90 | 10 | ||||
| 4 | 58 | 42 | ||||
| 8 | 51 | 49 | ||||
| 10.5 | 34 | 66 | ||||
| 11.5 | 10 | 90 | ||||
| 13 | 10 | 90 | ||||
| 13.5 | 90 | 10 | ||||
Assay performance characteristics
Reference intervals
Statistical analyses
Ethics
RESULTS
Performance of LC-MS/MS for nine steroid hormones
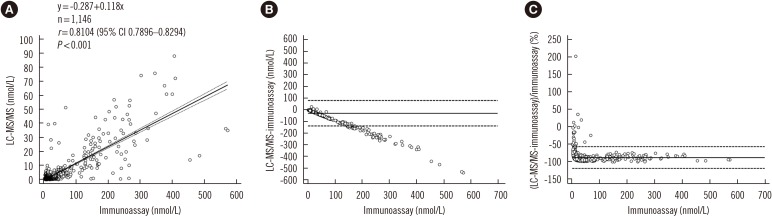 | Fig. 1Comparison of LC-MS/MS and immunoassay. Correlation (A), absolute difference (B), and percent bias (C) between LC-MS/MS and immunoassay results for 17α-hydroxyprogesterone in 1,146 dried blood spots from Korean neonates. The solid line in (A) is the best-fit regression line and dashed lines in (A) represent 95% confidence interval. Solid lines in (B) and (C) represent average differences, and dashed lines in (B) and (C) represent limit of agreement.Abbreviation: LC-MS/MS, liquid chromatography-tandem mass spectrometry.
|
Reference intervals and clinical application to 21-hydroxylase deficiency
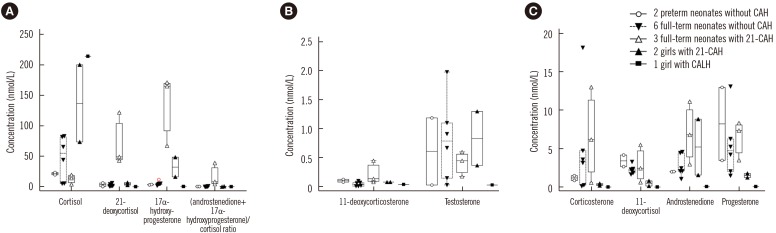 | Fig. 2Steroid profiles for 14 additional samples from neonates and children used to evaluate the clinical applicability of the LC-MS/MS method. Full-term neonates with 21-CAH (Δ) had distinctively low concentrations of cortisol, high concentrations of 21-deoxycortisol, 17α-hydroxyprogesterone, 11-deoxycorticosterone, and high values of (androstenedione+17α-hydroxyprogesterone)/cortisol ratio (A & B). Other steroid hormone concentrations in full-term neonates with 21-CAH were variable (C). Two children with 21-CAH had high concentrations of cortisol and 17α-hydroxyprogesterone, progesterone, and androstenedione. Concentrations of other steroids were not distinctive. One child with CALH had high concentration of cortisol and low concentrations of other steroids.Abbreviations: CAH, congenital adrenal hyperplasia; 21-CAH, congenital adrenal hyperplasia caused by 21-hydroxylase deficiency; CALH, congenital adrenal lipoid hyperplasia due to the StAR mutation; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
|
Table 2
Reference intervals (nmol/L) for nine steroid hormones in dried blood spots from Korean preterm and full-term neonates
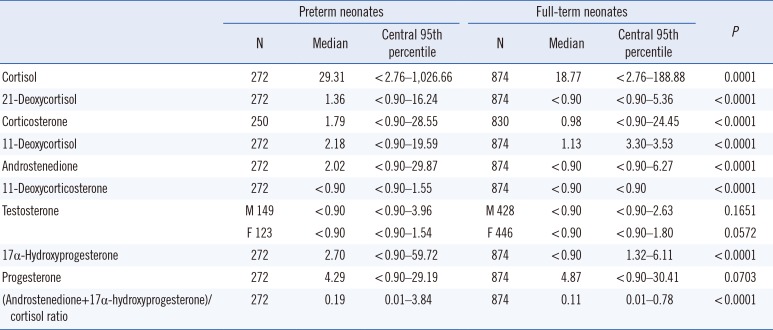
Table 3
Reference intervals (nmol/L) of nine steroid hormones in dried blood spots from Korean preterm neonates and comparisons with previous studies
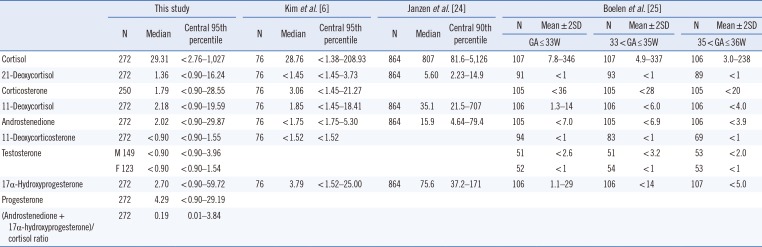
| This study | Kim et al. [6] | Janzen et al. [24] | Boelen et al. [25] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Central 95th percentile | N | Median | Central 95th percentile | N | Median | Central 90th percentile | N | Mean±2SD | N | Mean±2SD | N | Mean±2SD | |
| GA≤33W | 33<GA≤35W | 35<GA≤36W | |||||||||||||
| Cortisol | 272 | 29.31 | <2.76–1,027 | 76 | 28.76 | <1.38–208.93 | 864 | 807 | 81.6–5,126 | 107 | 7.8–346 | 107 | 4.9–337 | 106 | 3.0–238 |
| 21-Deoxycortisol | 272 | 1.36 | <0.90–16.24 | 76 | <1.45 | <1.45–3.73 | 864 | 5.60 | 2.23–14.9 | 91 | <1 | 93 | <1 | 89 | <1 |
| Corticosterone | 250 | 1.79 | <0.90–28.55 | 76 | 3.06 | <1.45–21.27 | 105 | <36 | 105 | <28 | 105 | <20 | |||
| 11-Deoxycortisol | 272 | 2.18 | <0.90–19.59 | 76 | 1.85 | <1.45–18.41 | 864 | 35.1 | 21.5–707 | 106 | 1.3–14 | 106 | <6.0 | 106 | <4.0 |
| Androstenedione | 272 | 2.02 | <0.90–29.87 | 76 | <1.75 | <1.75–5.30 | 864 | 15.9 | 4.64–79.4 | 105 | <7.0 | 105 | <6.9 | 106 | <3.9 |
| 11-Deoxycorticosterone | 272 | <0.90 | <0.90–1.55 | 76 | <1.52 | <1.52 | 94 | <1 | 83 | <1 | 69 | <1 | |||
| Testosterone | M 149 | <0.90 | <0.90–3.96 | 51 | <2.6 | 51 | <3.2 | 53 | <2.0 | ||||||
| F 123 | <0.90 | <0.90–1.54 | 52 | <1 | 54 | <1 | 53 | <1 | |||||||
| 17α-Hydroxyprogesterone | 272 | 2.70 | <0.90–59.72 | 76 | 3.79 | <1.52–25.00 | 864 | 75.6 | 37.2–171 | 106 | 1.1–29 | 106 | <14 | 107 | <5.0 |
| Progesterone | 272 | 4.29 | <0.90–29.19 | ||||||||||||
| (Androstenedione + 17α-hydroxyprogesterone)/cortisol ratio | 272 | 0.19 | 0.01–3.84 | ||||||||||||
*To convert ng/mL to nmol/L for data comparison, multiplication factors of 2.76 for cortisol, 2.89 for 21-deoxycortisol, corticosterone, and 11-deoxycortisol, 3.49 for androstenedione, 3.03 for 11-deoxycorticosterone and 17α-hydroxyprogesterone, and 3.67 for testosterone were applied [26].
Abbreviations: GA, gestational age; W, week.
Table 4
Reference intervals (nmol/L) of nine steroid hormones in dried blood spots from Korean full-term neonates and comparisons with previous studies
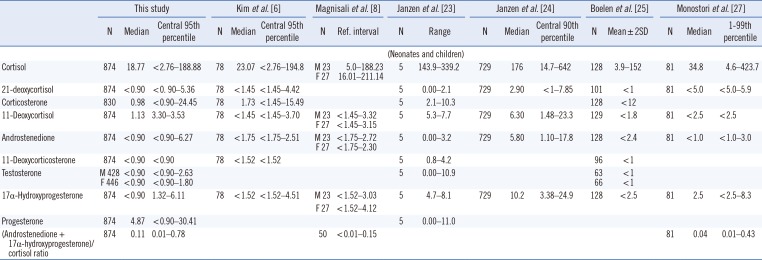
| This study | Kim et al. [6] | Magnisali et al. [8] | Janzen et al. [23] | Janzen et al. [24] | Boelen et al. [25] | Monostori et al. [27] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Central 95th percentile | N | Median | Central 95th percentile | N | Ref. interval | N | Range | N | Median | Central 90th percentile | N | Mean±2SD | N | Median | 1–99th percentile | |
| (Neonates and children) | ||||||||||||||||||
| Cortisol | 874 | 18.77 | <2.76–188.88 | 78 | 23.07 | <2.76–194.8 | M 23 | 5.0–188.23 | 5 | 143.9–339.2 | 729 | 176 | 14.7–642 | 128 | 3.9–152 | 81 | 34.8 | 4.6–423.7 |
| F 27 | 16.01–211.14 | |||||||||||||||||
| 21-deoxycortisol | 874 | <0.90 | <0.90–5.36 | 78 | <1.45 | <1.45–4.42 | 5 | 0.00–2.1 | 729 | 2.90 | <1–7.85 | 101 | <1 | 81 | <5.0 | <5.0–5.9 | ||
| Corticosterone | 830 | 0.98 | <0.90–24.45 | 78 | 1.73 | <1.45–15.49 | 5 | 2.1–10.3 | 128 | <12 | ||||||||
| 11-Deoxycortisol | 874 | 1.13 | 3.30–3.53 | 78 | <1.45 | <1.45–3.70 | M 23 | <1.45–3.32 | 5 | 5.3–7.7 | 729 | 6.30 | 1.48–23.3 | 129 | <1.8 | 81 | <2.5 | <2.5 |
| F 27 | <1.45–3.15 | |||||||||||||||||
| Androstenedione | 874 | <0.90 | <0.90–6.27 | 78 | <1.75 | <1.75–2.51 | M 23 | <1.75–2.72 | 5 | 0.00–3.2 | 729 | 5.80 | 1.10–17.8 | 128 | <2.4 | 81 | <1.0 | <1.0–3.0 |
| F 27 | <1.75–2.30 | |||||||||||||||||
| 11-Deoxycorticosterone | 874 | <0.90 | <0.90 | 78 | <1.52 | <1.52 | 5 | 0.8–4.2 | 96 | <1 | ||||||||
| Testosterone | M 428 | <0.90 | <0.90–2.63 | 5 | 0.00–10.9 | 63 | <1 | |||||||||||
| F 446 | <0.90 | <0.90–1.80 | 66 | <1 | ||||||||||||||
| 17α-Hydroxyprogesterone | 874 | <0.90 | 1.32–6.11 | 78 | <1.52 | <1.52–4.51 | M 23 | <1.52–3.03 | 5 | 4.7–8.1 | 729 | 10.2 | 3.38–24.9 | 128 | <2.5 | 81 | 2.5 | <2.5–8.3 |
| F 27 | <1.52–4.12 | |||||||||||||||||
| Progesterone | 874 | 4.87 | <0.90–30.41 | 5 | 0.00–11.0 | |||||||||||||
| (Androstenedione + 17α-hydroxyprogesterone)/cortisol ratio | 874 | 0.11 | 0.01–0.78 | 50 | <0.01–0.15 | 81 | 0.04 | 0.01–0.43 | ||||||||||
*To convert ng/mL to nmol/L for data comparison, multiplication factors of 2.76 for cortisol, 2.89 for 21-deoxycortisol, corticosterone, and 11-deoxycortisol, 3.49 for androstenedione, 3.03 for 11-deoxycorticosterone and 17α-hydroxyprogesterone, 3.18 for progesterone, and 3.67 for testosterone were applied [26].
Abbreviations: F, female; GA, gestational age; LLOQ, lower limit of quantification; M, male; W, week.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download