Abstract
Extra-articular tenosynovial giant cell tumor (TS-GCT) in retropharyngeal space is a rare case. We found only two case reports in the literature, in which one was located in retropharynx or prevertebral space of the cervical spine. We describe a rare case of TS-GCT in the retropharynx, which was initially misdiagnosed as oropharyngeal cancer. Furthermore, we want to assure that extraarticular diffuse type TS-GCT should be considered in the differential diagnosis of lesions showing low signal intensity in MRI scan.
Tenosynovial giant cell tumor (TS-GCT), also known as giant cell tumor of the tendon sheath or pigmented villonodular synovitis is a benign lesion of the tendon sheath, bursae, and synovium (1). Extraarticular TS-GCT is commonly located in the tendon sheath, bursa, and periarticular soft tissues; however, on rare occasions, it may be located in other regions with no association with the synovial tissues, making diagnosis a challenging task (2). The occurrence of TS-GCT in the retropharynx is a very rare case. In the literature, the presence of TS-GCT in the retropharynx has been reported in only one case (3).
In this report, we describe a rare case of TS-GCT in the retropharynx, which was initially misdiagnosed as oropharyngeal cancer.
A 70-year-old man visited the otorhinolaryngology department because of an accidentally discovered mass in the right posterior oropharynx. On laryngoscopy, a bulging mass with smooth margins was observed on the posterior oropharyngeal wall (Fig. 1). For further evaluation, computed tomography (CT) and magnetic resonance imaging (MRI) scans were performed. The CT scan depicted a heterogeneously enhancing mass with smooth margins in the right retropharyngeal space at the level of C2. Posteriorly, the mass appeared to have eroded the anterior portion of the C2 vertebral body and odontoid process and was abutting with the inferior margin of the anterior atlantoaxial (AA) joint (Fig. 2). An MR image of the oropharynx depicted a mass with hypointense signal intensity on T1- and T2-weighted imaging and heterogeneous enhancement on enhanced T1-weighted imaging (Fig. 3). As a result, the lesion was diagnosed as oropharyngeal cancer.
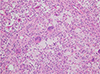
The patient underwent partial excisional biopsy via an intraoral approach. A histopathological examination revealed numerous foamy histiocytes and scattered multinucleated giant cells, suggestive of a diffuse type tenosynovial giant cell tumor (Fig. 4). On immunohistochemical study, the lesion exhibited expression of CD68 and CD45.
The patient did not undergo complete surgical excision and continued treatment with monoclonal antibodies (denosumab) for a year. On follow-up enhanced CT scan, the size of the mass was slightly decreased.
TS-GCT, also known as pigmented villonodular synovitis, is a benign soft tissue tumor that arises from the synovium of the joint, bursa, and tendon sheath (12).
TS-GCT can be classified as localized and diffuse types, based on the pattern of growth, and as intraarticular and extraarticular types, based on the location. The intraarticular type is more common than the extraarticular type. In our case, as the mass was abutting with the inferior border of the atlantoaxial joint, the intraarticular TS-GCT with the extraarticular extension was considered. However, as the center of the mass was in the anterior aspect of the joint and virtually did not involve the joint, the probability was towards the extraarticular type than the intraarticular type with extraarticular extension.
The extraarticular type of TS-GCT is of rare occurrence and was first described by Jaffe et al. (4); it is commonly localized and exhibits a benign clinical course. As it arises from the tendon sheath, it is also called as ‘the GCT of the tendon sheath’ (2).
The diffuse type TS-GCT is classified as a ‘fibrohistiocytic tumor’ in the World Health Organization system of classification of bone and soft-tissue tumors, 2013. Unlike the localized type, diffuse type TS-GCT is usually aggressive and often shows multiple recurrences (2).
On imaging, the extraarticular diffuse type TS-GCT is commonly located in the periarticular soft tissues with the involvement of adjacent joints, but on rare occasions, it can be characterized by the presence of an infiltrative soft tissue mass, without the involvement of the adjacent joint (25).
In most of the reports, extraarticular diffuse type TS-GCT is demonstrated as an ill-defined and infiltrative mass; however, it may also present as a well-defined mass, as in the present case. Unlike localized TS-GCT, the diffuse type is more frequently invasive to the adjacent soft tissue and bone (6).
On T1- and T2-weighted MR images, TS-GCT predominantly exhibits low signal intensity, reflective of hemosiderin deposition (27), but it may also show variable signal intensities, depending on the composition of the lesion and the relative proportion of hemosiderin, lipid, fibrous tissue, cyst formation, and cellular elements (1).
Pathologically, the extraarticular variant typically lacks a villous pattern. Grossly, a multifocal alternative pattern of white, yellowish and brownish lesions is common. Microscopically, TS-GCT is composed of proliferative synovial-like mononuclear cells, multinucleated giant cells, compact fibrous stromal cells, foamy and hemosiderin-laden cells etc. (46).
Diffuse type TS-GCT is composed of a striking vascularization pattern with thin-walled, slit-like, and partially hyalinized small blood vessels, including giant hemosiderin granules. Immunohistochemical analysis has revealed the expression of CD 68, CD3, and calretinin in diffuse-type TS-GCT (58).
Until date, only one case of extraarticular diffuse type TS-GCT in the retropharyngeal space has been reported and it involved the retropharyngeal space from the posterior pharyngeal wall to the level of the epiglottis. Total tumor excision and postoperative radiation therapy were performed, and no recurrence was observed (3).
Because of its rarity, extraarticular diffuse type TS-GCT is often not included among the differential diagnoses, and the lesion may often be misdiagnosed. In our case, TS-GCT was not considered during imaging evaluation (6).
The recommended treatment for TS-GCT is complete resection if possible because of the aggressive nature (9). For recurrent or unresectable lesions, moderate-dose adjuvant radiotherapy could provide good local control. However, recently, monoclonal antibodies such as imatinib, having activity against CSF1 receptor, have been used for treating TS-GCT in patients with recurrent or inoperable lesions (710). Most of the tumors appear to be clonal, neoplastic proliferations driven by CSF1 production of the neoplastic cells. They could be treated by a tyrosine kinase receptor inhibitor, such as imatinib thus demonstrating early success in the treatment (6).
In conclusion, tenosynovial giant cell tumor can involve extremely rare locations, like the retropharynx. In such cases, TS-GCT is often not included among the differential diagnoses because of the rarity of its occurrence. However, extraarticular diffuse type TS-GCT should be considered in the differential diagnosis of lesions that show low signal intensity appropriate for a giant cell tumor.
Figures and Tables
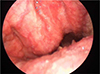 | Fig. 1Laryngoscopy reveals a smooth margined bulging contour mass lesion in the posterior oropharynx. |
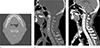 | Fig. 2Cervical spine CT scan bone setting axial (a), sagittal (b) and soft tissue setting with enhancement (c). (a, b) The axial/reformatted sagittal bone algorithm shows bony lytic lesion with partially very thin sclerotic margin involving C2 odontoid process and vertebral body, abutting with the inferior margin of the anterior atlantoaxial (AA) joint. (c) Sagittal contrast-enhanced CT scan shows a lytic, heterogeneously enhancing soft-tissue density mass involving the C2 extending to prevertebral space, as expected from a mass originating in the prevertebral space. |
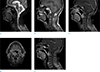 | Fig. 3T1 sagittal (a), T2 fat suppression sagittal (b), T1 enhancement sagittal (c) (e), axial (d). (a) Sagittal T1 weighted image shows heterogeneously intermediate signal intensity mass involving the odontoid process and vertebral body of C2 and abutting with the inferior margin of the anterior atlantoaxial (AA) joint. (b) Sagittal T2 weighted image with fat-suppression shows markedly hypointense mass. (c) T1 weighted image with contrast enhancement sagittal view, (d) axial view shows heterogeneous enhancement and no evidence of perilesional extension. (e) Enhanced T1 weighted image sagittal view shows the mass abutting with the inferior border of the atlantoaxial joint but nearly not involving the joint. |
References
1. Koontz NA, Quigley EP, Witt BL, Sanders RK, Shah LM. Pigmented villonodular synovitis of the cervical spine: case report and review of the literature. BJR Case Rep. 2016; 2:20150264.

2. Rateb K, Hassen BG, Leila A, Faten F, Med Samir D. Giant cell tumor of soft tissues: a case report of extra-articular diffuse-type giant cell tumor of the quadriceps. Int J Surg Case Rep. 2017; 31:245–249.

3. Paulino AF, Spiro RH, O'Malley B, Huvos AG. Giant cell tumour of the retropharynx. Histopathology. 1998; 33:344–348.

4. Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. A discussion of synovial and bursal equivalents of the tenosynovial lesion commonly denoted as xanthorna, xanthogranuloma, giant cell tumor or myeloplaxoma of the tendon sheath, with some consideration of this tendon sheath lesion itself. Arch Pathol. 1941; 31:731–765.
5. Savvidou OD, Mavrogenis AF, Sakellariou VI, Chloros GD, Sarlikiotis T, Papagelopoulos PJ. Extra-articular diffuse giant cell tumor of the tendon sheath: a report of 2 cases. Arch Bone Jt Surg. 2016; 4:273–276.
6. Lucas DR. Tenosynovial giant cell tumor: case report and review. Arch Pathol Lab Med. 2012; 136:901–906.

7. Ravi V, Wang W, Araujo DM, et al. Imatinib in the treatment of tenosynovial giant-cell tumor and pigmented villonodular synovitis. J Clin Oncol. 2010; 28(15s):10011.

8. Kuhnen C, Muller KM, Rabstein S, Kasprzynski A, Herter P. Tenosynovial giant cell tumor. Pathologe. 2005; 26:96–110.
9. Dingle SR, Flynn JC, Flynn JC Jr, Stewart G. Giant-cell tumor of the tendon sheath involving the cervical spine. A case report. J Bone Joint Surg Am. 2002; 84-A:1664–1667.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download