Abstract
A 66-year-old woman was referred for treatment of incidental detection of two intracranial aneurysms. Time-of-flight MR angiography (TOF MRA) revealed two aneurysms at the M1 segment of the right middle cerebral artery, and clinoid segment of left internal carotid artery, respectively. On digital subtraction angiography, there was a saccular aneurysm on the left internal carotid artery, but the other aneurysm was not detected on the right middle cerebral artery. Based on comprehensive review of imaging findings, organized thrombosed aneurysm was judged as the most likely diagnosis. In the presented report, magnetization transfer (MT) pulse to TOF MRA was used, to differentiate aneurysm-mimicking lesion on TOF MRA. We report that MT technique could be effective in differentiating true aneurysm, from possible T1 high signal artifact on TOF MRA.
Magnetization transfer contrast (MTC) in magnetic resonance imaging (MRI) is the result of interaction of protons between bulk water and macromolecules of tissue (1). Since the different degree of magnetization transfer (MT) caused by different macromolecular compositions from different tissues, can generate tissue contrast according to well-defined biophysical and biochemical properties, MTC has been expected to improve contrast in MRI in various fields (12345). For example, an off-resonance MTC pulse was added for further saturation of background tissue, and to improve vessel conspicuity in time-of-flight magnetic resonance angiography (TOF MRA) (4). Consequently, MTC imaging is mainly used in TOF MRA, to improve signal contrast between blood and other tissues (24567). Although many of the potential applications of MTC could be promising in clinical fields, MTC applied TOF MRA (MTC TOF MRA) has not been extensively applied to clinical fields unfortunately, and are considered works in progress.
In this study, we applied MTC TOF MRA for a 66-year-old woman with aneurys-mmimicking lesion on TOF MRA. Diagnostic pitfalls during evaluation of 3D TOF MRA could include any phenomena related with high-signal structures mimicking vascular abnormalities, such as lipoma, aneurysm, subacute thrombus, and circumferential calcification (8910). Among them, lipoma is the most common lesion that can be differentiated by phase shift TOF MRA (11). However chemical shift artifact was not observed around the lesion in the patient. Our hypothesis was that possible false positive lesion could reveal different MTC TOF MRA, due to different MT according to macromolecular compositions. We report that MTC TOF MRA could be effective in differentiating true aneurysm, from possible T1 high signal artifact on TOF MRA.
A 66-year-old woman was referred for treatment of two incidentally detected intracranial aneurysms; one was at the M1 segment of right middle cerebral artery, and the other at the clinoid segment of left internal carotid artery, on screening MRI including TOF MRA. On digital subtraction angiography (DSA) for diagnostic confirmation and pretreatment evaluation, there was a saccular aneurysm on the left internal carotid artery (ICA), but the other aneurysm was invisible, on the right middle cerebral artery (Fig. 1).
TOF MRA was performed on a GE Discovery MR750 3T MR machine (GE Healthcare, Waukesha, WI, USA), using 3D-TOF technique (repetition time [TR]: 20 msec, echo time [TE]: 3.1 msec, flip angle: 18, field of view [FOV]: 159 mm × 199 mm, matrix: 416 × 416, slice thickness: 0.8 mm, number of excitation [NEX]: 1). To evaluate of the effect of chemical shift artifact, out-of-phase (TE, 5.7 msec) TOF images were obtained. Phase shift TOF MRA did not reveal chemical artifact around the hyperintense lesion. For MT imaging, TOF MRI with a preparing saturation pulse (1200 Hz off-resonance, Fermi, MR pulse type, 950° flip-angle, 8 msec) was acquired. MTC subtraction (MTCS) was calculated using the equation: MTCS = MTon − MToff, where MTon is the image with MT and MToff is the image from the same sequence without a saturation pulse. MTCS angiography (MTCSA) was reconstructed using MIP to MTC map. MTCSA was similar to the result of DSA (Fig. 2). MTR, that represented signal reduction after MT saturation, was calculated pixel by pixel with the equation: MTR = (MToff − MTon) /MToff. MTR mapping was calculated with Image J program (1.47q, NIH, USA). MTR of pitfall in the right middle cerebral artery, and true lesion in the left internal carotid, was 0.170 ± 0.230, and 0.421 ± 0.209 respectively. CT revealed rim calcification (Hounsfield unit = 134.2 ± 30.2) without iodine contrast medium, filling in the vicinity of the right middle cerebral artery (Fig. 3). Aneurysm in the left ICA was embolized with detachable coil.
Although DSA has been regarded as the gold standard for diagnosing intracranial aneurysms, TOF MRA is frequently used as a non-invasive diagnostic option for screening purposes, and an alternative to DSA for operative management (1213). TOF MRA is highly sensitive and specific for diagnosing aneurysms larger than 5 mm (1014). However, diagnostic pitfalls of TOF MRA could be a main potential limitation, that arises from high signal intensity structures mimicking vascular abnormalities on TOF source images, or maximum-intensity-projection reconstructions. Potential pitfalls in the vicinity of the intracranial artery include lesions such as lipomas, aneurysms, subacute thrombus, and circumferential calcification, as well as normal anatomies such as complex flow-related artifacts, and neurohypophysis of the pituitary gland (8915). So, assessment of aneurysms using TOF MRA requires careful evaluation of axial source images, as well as multiplanar reconstructions, for lesions smaller than 3 mm (1617). Although lipomas are generally clearly characteristic on cross-sectional imaging, lesions smaller than 5 mm vicinity to an intracranial artery may be misinterpreted as aneurysms on TOF MRA, if additional T1 fat-suppressed series or CT images are unavailable for definite diagnosis. Edge artifact by in-phase and India ink artifact by out-of-phase chemical shift are characteristic findings of lipomas on TOF source images (18). In this patient, diagnosis of intracranial lipoma was excluded on MTC MRA, due to absence of a chemical shift artifact suggesting fat component. Instead, the T1 hyperintense lesion could be alleged to be a thrombosed aneurysm, due to its feature of CT such as wall calcification, and lack of luminal contrast filling (19). To the best of our knowledge, this is the first report that thrombosed aneurysm may be visualized on TOF MRA, but not revealed on MTon − MToff. MTR value of thrombosed aneurysm was about half of that of non-thrombosed aneurysm, suggesting significant signal reduction after MT saturation. More clinical study with a large sample would be necessary, for confirming this hypothesis. In this report, MT pulse was used to differentiate aneurysm-mimicking lesion on TOF MRA. Initially, an off-resonance MTC pulse was added to an RF spoiled gradient-echo sequence, improve conspicuity of the vessel in TOF MRA by saturation of background tissue (4). In this case, better conspicuity of lenticulostriate arteries noted on MTon angiography, as we expected. Interestingly, we also found that the aneurysm-mimicking lesion at the right middle cerebral artery on TOF MRA, was not revealed on MTCSA, similar to that of DSA.
A recent study revealed that ex vivo plaque imaging using MTC MR, discriminated the component of the plaque from tissue (20). According to their results, recent hematoma and thick collagen fiber had higher MTR, than thin collagen fiber or calcification. In this study, MTR of aneurysm-mimicking lesion at the right middle cerebral artery was 0.170 ± 0.230, considered as a mixture of thin fiber with wall calcification. The lesion also revealed a high-density wall, with relatively low-density core on CT. So, the most likely diagnosis based on morphology and MTR, is a thrombosed aneurysm with bright T1 signal from old blood products. The most worrisome situation is that it could be partially thrombosed aneurysm of the lesion or recanalized aneurysm, that may request complicated treatment options, including close observation and endovascular surgery. MTCSA confirmed no blood flow into the lesion. In addition to visual assessment, MTR calculated from MTC values, allowed characterization of the lesion, that could be used as a surveillance tool in follow-up imaging.
Despite improved vessel conspicuity, MT applied TOF MRA was not used widely as an advanced vascular imaging technique in clinical application, and it is not necessary to apply for clinical TOF MRA with screening purpose. The other limitation of MTC was increase in minimum TR, by adding several milliseconds of off resonance MTC pulse (2). However, there is little solution other than MTC, for differentiating false positive lesion in clinical field. In this case report, it was focused on a technical approach with intention of problem solving, for false positive intracranial aneurysm, other than lesion containing lipid component on TOF MRA using MTCA.
In summary, this case report demonstrated that non-lipomatous false positive lesion and intracranial aneurysm on TOF MRA, were differentiated by using MTCA with capability of the estimation of tissue property, as well as higher signal contrast. Consequently, MT technique could be an alternative technique in characterizing non-lipomatous false positive lesion. In conclusion, we report that use of MT technique could be effective in differentiating true aneurysm, among high intensity lesions on TOF MRA. To our knowledge, this is first report of the potential of MTCA, in assessing pitfalls on intracranial TOF MRA. Although MTCA has been rarely applied to clinical fields until now, the technique could be suggested as promising methodology for vascular imaging.
Figures and Tables
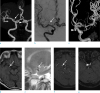 | Fig. 166-year-old woman with a diagnostic pitfall on TOF MR angiography. TOF MRA (a) shows two intracranial aneurysms; one is located at the M1 segment of right middle cerebral artery (MCA; arrow) and the clinoid segment of left internal carotid artery (ICA; arrowhead). Aneurysm was invisible (arrow) digital subtraction angiography (b). In contrast, saccular aneurysm from the left ICA is noted on 3D Rotational angiography (c, arrowhead). The lesion at the vicinity of the right MCA shows high signal intensity (arrow) on T1 (d) and low signal intensity (arrow) on T2 weight MR image (e). High signal intensity lesions suggestive aneurysms (arrow) at the vicinity of right MCA (f) and ophthalmic segment of left ICA (arrowhead) (g) are noted on the source images of TOF MRA. |
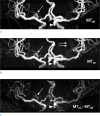 | Fig. 2Magnetization transfer contrast angiography. As compared with an aneurysm (arrow) on MToff (a), better conspicuity of lenticulostriate arteries (double arrows) is noted on MTon angiography (b). MTC subtraction MRA (c) as a result of subtraction of MTon (b) from MToff (a) shows no aneurysm (arrow) in the right middle cerebral artery, contrary to well definition of aneurysm (arrowhead) at right ICA. |
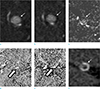 | Fig. 3Phase shift TOF MRA (a: in-phase, b: out-of-phase) did not showed chemical artifact around small hyperintense lesion (arrows) in the right middle cerebral artery. Source image of magnetization transfer contrast MRA (c) shows low signal intensity of pitfall (arrow). MTR of pitfall (open arrow) (d) and true lesion (open arrow) (e) was 0.170 ± 0.230, and 0.421 ± 0.209, respectively. CT (f) shows high-density wall (Hounsfield unit = 134.2 ± 30.2) with relatively low-density core (arrow) (Hounsfield unit = 64.4 ± 20.2) on the center of lesion. |
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. NRF-2012R1A1A4A01005117).
References
1. Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989; 10:135–144.
2. Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001; 14:57–64.

3. Pike GB, Hu BS, Glover GH, Enzmann DR. Magnetization transfer time-of-flight magnetic resonance angiography. Magn Reson Med. 1992; 25:372–379.

4. Edelman RR, Ahn SS, Chien D, et al. Improved time-of-flight MR angiography of the brain with magnetization transfer contrast. Radiology. 1992; 184:395–399.

5. Atkinson D, Brant-Zawadzki M, Gillan G, Purdy D, Laub G. Improved MR angiography: magnetization transfer suppression with variable flip angle excitation and increased resolution. Radiology. 1994; 190:890–894.

6. Nezafat R, Han Y, Peters DC, et al. Coronary magnetic resonance vein imaging: imaging contrast, sequence, and timing. Magn Reson Med. 2007; 58:1196–1206.

7. Lin W, Tkach JA, Haacke EM, Masaryk TJ. Intracranial MR angiography: application of magnetization transfer contrast and fat saturation to short gradient-echo, velocity-compensated sequences. Radiology. 1993; 186:753–761.

8. Anderson CM, Saloner D, Tsuruda JS, Shapeero LG, Lee RE. Artifacts in maximum-intensity-projection display of MR angiograms. AJR Am J Roentgenol. 1990; 154:623–629.

9. Wilcock DJ, Jaspan T, Worthington BS. Problems and pitfalls of 3-D TOF magnetic resonance angiography of the intracranial circulation. Clin Radiol. 1995; 50:526–532.

10. Wermer MJ, van der Schaaf IC, Velthuis BK, Majoie CB, Albrecht KW, Rinkel GJ. Yield of short-term follow-up CT/MR angiography for small aneurysms detected at screening. Stroke. 2006; 37:414–418.

11. Soila KP, Viamonte M Jr, Starewicz PM. Chemical shift misregistration effect in magnetic resonance imaging. Radiology. 1984; 153:819–820.

12. Sankhla SK, Gunawardena WJ, Coutinho CM, Jones AP, Keogh AJ. Magnetic resonance angiography in the management of aneurysmal subarachnoid haemorrhage: a study of 51 cases. Neuroradiology. 1996; 38:724–729.

13. Litt AW. MR angiography of intracranial aneurysms: proceed, but with caution. AJNR Am J Neuroradiol. 1994; 15:1615–1616.
14. Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000; 123(Pt 2):205–221.

15. Okahara M, Kiyosue H, Yamashita M, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke. 2002; 33:1803–1808.
16. Horikoshi T, Fukamachi A, Nishi H, Fukasawa I. Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology. 1994; 36:203–207.

17. Adams WM, Laitt RD, Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol. 2000; 21:1618–1628.
18. Kemmling A, Noelte I, Gerigk L, Singer S, Groden C, Scharf J. A diagnostic pitfall for intracranial aneurysms in time-of-flight MR angiography: small intracranial lipomas. AJR Am J Roentgenol. 2008; 190:W62–W67.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download