Abstract
Superficial siderosis of the central nervous system (CNS) is a progressive and debilitating neurological disease manifesting sensorineural hearing loss, cerebellar ataxia, and pyramidal tract signs. Chronic extravasation of blood into the subarachnoid space results in the accumulation of hemoglobin derivate in the subpial layer of the CNS, which is toxic to the neural tissues. Craniopharyngioma is a benign third ventricle tumor, which rarely presents with tumor bleeding. We report a rare case of superficial siderosis associated with craniopharyngioma with intratumoral hemorrhage in a patient with no history of prior trauma or CNS surgery.
Superficial siderosis of the central nervous system (CNS) is a rare condition caused by deposition of hemosiderin in the brain and spinal cord. The typical clinical manifestation of superficial siderosis includes sensorineural hearing loss, gait ataxia and pyramidal signs (1). The chronic existence of bleeding source in the subarachnoid space results in the deposition of hemosiderin in the subpial layer of the CNS (2). The source of bleeding is variable, including vascular malformations, trauma, CNS tumors, and CNS surgery (13).
Craniopharyngioma is a rare brain tumor and intratumoral hemorrhage is one of the uncommon manifestations of craniopharyngioma (4). Also, superficial siderosis associated with craniopharyngioma is rarely reported in previous studies (3). We report a rare case superficial siderosis associated with craniopharyngioma with intratumoral hemorrhage.
A 50-year-old man presented to the emergency department with dizziness and headache. He had intermittent dizziness and headache for the past three years, and the symptoms aggravated two weeks back. The patient had medications for hypertension and panic disorder. He had no history of the traumatic event. Neurologic examination revealed no significant neurologic deficit. Computed tomography (CT) revealed the presence of about 2.3 cm sized isodense lesion with an internal hyperdense component in suprasellar cistern, and the lesion showed heterogenous contrast enhancement on following contrast-enhanced brain CT (Fig. 1). Brain MRI revealed the location of the mass in the anterior aspect of the third ventricle involving the hypothalamus and medial aspect of bilateral thalami. There was no evidence of hydrocephalus. The mass showed heterogeneous high signal intensity (SI) on T2-weighted images (T2WI) with strong and heterogeneous contrast enhancement. Peritumoral T2 high SI involved optic chiasm but spared pituitary fossa. Focal T2WI dark SI lesions with blooming artifact on gradient-echo T2*-weighted images (GRE T2*WI) were noted in the tumor, suggesting intratumoral hemorrhage. The preoperative diagnosis was hypothalamic-chiasmatic glioblastoma multiforme. The result of single-voxel spectroscopy supported this diagnosis, as prominent lactate peak presented, although there was noise in basal spectroscopy due to intratumoral hemorrhage. However, the tumor did not show diffusion restriction or extensive peritumoral T2WI high SI, and the findings were not consistent with the findings of glioblastoma multiforme. On T2WI and GRE T2*WI, diffuse linear low SI was noted along bilateral Sylvian fissures, frontotemporal and cerebellar sulci, suggesting superficial hemosiderosis (Fig. 2). A small amount of intraventricular hemorrhage was noted in both the lateral ventricles (Fig. 2). The third ventricle tumor was considered as the bleeding focus, as there was no other evidence of focus of intracranial hemorrhage. No significant diffusion restriction was noted in the tumor. The endocrinologic blood test revealed subclinical hypothyroidism, but no other significant finding was reported. Serum electrolyte levels were within the normal range. The patient underwent tumor resection with pterional-transsylvian and subfrontal approach. On the operation field, the tumor was hard and gray in color and located in the third ventricle. Arachnoid membrane showed dark brown color. The pathologic diagnosis of the tumor was papillary type craniopharyngioma. Also, hemosiderin pigment was reported on a pathologic report of meninges. Cerebrospinal fluid (CSF) study revealed no evidence of the presence of malignant cells. After 14 days of the surgery, the patient showed a decrease in mentality and CT revealed diffuse brain swelling with subfalcine brain herniation. After two times of decompressive craniectomy, brain swelling and brain herniation improved gradually. However, during the postoperative care period, the patient developed 38 degrees of fever with an elevation of C-reactive protein. After application of vancomycin and cefepime for coverage of hospital-acquired pneumonia and operation site infection for 15 days, a rapid decrease in glomerular filtration rate was observed. Therefore, the patient underwent continuous renal replacement therapy for acute kidney injury. Also, the patient underwent follow-up CT for evaluation of decreased mental status, which revealed the marked progression of brain swelling. On the subsequent day (42nd day of admission), the patient expired as the blood pressure was not maintained even after application of vasopressin.
Recurrent and prolonged hemorrhage in subarachnoid space leads to superficial siderosis of CNS. CNS tissues convert heme to free iron, ferritin, and hemosiderin in CSF and accumulation of these hemoglobin derivates in the leptomeninges results in destruction and demyelination of CNS tissues (2). Patients usually manifest sensorineural hearing loss, gait ataxia, dementia, and myelopathy, but the number of presymptomatic cases has increased with the widespread use of MRI (56). The source of chronic bleeding is quite variable. Head or back trauma and prior intradural surgery are well-known risk factors (6). Other etiologies include CNS tumor, nerve root injury, vascular malformation and amyloid angiopathy (56). MRI is the best imaging modality for the diagnosis of superficial siderosis and detection of bleeding focus (16). T2WI low SI along with the brain sulcus, spinal cord, and cranial nerves is the pathognomonic finding of superficial siderosis. GRE T2*WI exhibits a higher sensitivity for the detection of hemosiderin deposition, because of magnetic susceptibility effects of blood-degradation products (1). Often, superficial siderosis is accompanied by cerebellar atrophy and spinal cord atrophy. CT and CT myelography may provide additional information, such as the presence of a bony defect associated with trauma a or prior surgery and dural defect associated with pseudomeningocele (6). Cerebral angiography and spinal angiogram are rarely beneficial for the detection of an occult bleeding focus, which might be due to the slow flow and intermittent character of the bleeding source (1).
For differential diagnosis of anterior third ventricle masses in adults, hypothalamic-chiasmatic masses should be considered, including craniopharyngioma, hypothalamic-chiasmatic glioma, chordoid glioma, lymphoma, and metastases (7). Papillary type craniopharyngioma is an uncommon subtype of craniopharyngioma, which usually occurs in middle-aged adults. Most of the lesions are suprasellar, spherical and predominantly solid mass and rarely show calcification (8). Spontaneous tumor bleeding is a very uncommon finding of craniopharyngioma (9). Hypothalamic-chiasmatic glioma also appears as third ventricle tumor, which shows a more aggressive clinical course in adults (8). Larger tumors tend to be heterogeneous with both cystic and solid components (7). Chordoid glioma is another anterior third ventricle neoplasm containing both glial and chordoid histologic component. Chordoid glioma appears as a well-defined, T1 iso SI, ovoid suprasellar mass with intense contrast enhancement, which displaces infundibulum posteriorly (7). It is often difficult to differentiate between these disease entities. Therefore, the final diagnosis usually depends on the pathologic diagnosis.
In our case, diffuse hemosiderin deposition along bilateral Sylvian fissures and cerebellum were compatible with typical findings of superficial siderosis. The patient did not have a history of trauma and intradural surgery and no definite evidences of occult head trauma were noted on brain CT and MRI. There was a solid mass in the third ventricle with acute hemorrhage on CT. Therefore, the tumor bleeding was a highly suspected bleeding focus of superficial siderosis. Our case report is the second reported case of superficial siderosis due to preoperative intratumoral hemorrhage of craniopharyngioma detected in a living patient worldwide. The first case was reported in Japan by Tosaka et al. (3) In both the cases, patients were healthy males in their 6th-7th decades. The pathologic type of craniopharyngiomas was papillary type in both the patients, and this result is similar to previously reported craniopharyngiomas with hemorrhage (9). Although the pathogenesis of intratumoral hemorrhage in craniopharyngioma is unclear, several hypotheses were suggested, such as degenerative changes and rupture of blood vessels walls in the craniopharyngioma and connective tissue, and the presence of numerous immature blood vessels (10). In the present case, it was not possible to detect pathologic findings associated with intratumoral hemorrhage due to the limitation of the specimen. In contrary to the patient in the first case report, our patient did not show hydrocephalus on images. Hydrocephalus is one of the complications of superficial siderosis, which is probably due to adhesion in subarachnoid space (5).
The mainstay of treatment for superficial siderosis is identification and ablation of the bleeding focus for prevention of progression of symptoms (6). Once symptoms occur, it is difficult to reverse CNS damage caused by hemosiderin deposition (3). Therefore, early diagnosis and timely treatment are important for the better outcome. Until now, several cases reported that chelation therapies, using desferrioxamine, deferiprone and trientine, steroids and lumbar CSF shunting are effective for improvement of symptoms (6). In cases of patients with non-specific symptoms and underlying disease such as brain tumor, like our case, it is important to inform the clinicians that existence of superficial siderosis might have caused the symptom, as the symptom may persist even after treatment of the underlying disease. In our patient, he had an intermittent headache and dizziness without neurologic deficit, and the symptoms were not specific for superficial siderosis. As the patient expired after the tumor removal surgery, we could not evaluate the response of the symptoms after the surgery. In conclusion, we report an extremely rare case of superficial siderosis with hemorrhagic craniopharyngioma in a 50-year-old man. As the patient had no history of head trauma or operation history on brain and spine, craniopharyngioma with intratumoral hemorrhage in the third ventricle was considered as the bleeding focus, which is a rare manifestation for craniopharyngioma. Our case report is worthwhile to review similar cases of hemorrhagic craniopharyngiomas and differential diagnoses for the third ventricle tumors. Also, our case demonstrated superficial siderosis of the CNS as a rare complication of hemorrhagic craniopharyngioma. Although superficial siderosis of the CNS is a very rare condition, it is associated with pathognomonic findings on brain MRI. Detection of the bleeding source is critical for treatment of superficial siderosis of the CNS. Therefore, radiologists should be aware of this rare condition and be able to suggest appropriate imaging modality for the evaluation of patients with superficial siderosis of the CNS.
Figures and Tables
Fig. 1
(a) Coronal non-enhanced computed tomography (CT) scan shows about 2.3 × 1.8 × 2.0 cm sized isodense suprasellar mass with the focal internal high attenuated area. (b) Contrast-enhanced CT scan demonstrates heterogenous contrast enhancement of the lesion.
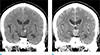
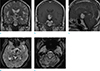
Fig. 2
(a) On coronal T2-weighted image (T2WI), the mass is in the third ventricle, without obstruction of foramen of Monro. No evidence of hydrocephalus is seen. The mass shows high signal intensity (SI) on T2WI and (b) strong, heterogeneous contrast enhancement. (c) On sagittal contrast-enhanced T1-weighted image, the mass is in the anterior aspect of the third ventricle, without involvement of pituitary fossa. (a) Marked T2 low SI foci is noted within the mass, which can be correlated with high density lesion in non-enhanced CT scan, and this finding suggests acute hemorrhage in the mass. (d, e) On axial gradient-echo T2*-weighted images, diffuse linear low SI is noted along bilateral Sylvian fissures and cerebellar sulci, suggesting superficial hemosiderosis. Small amount of intraventricular hemorrhage is noted in bilateral lateral ventricles.
References
1. Kumar N. Neuroimaging in superficial siderosis: an in-depth look. AJNR Am J Neuroradiol. 2010; 31:5–14.

2. Koeppen AH, Michael SC, Li D, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. 2008; 116:371–382.

3. Tosaka M, Sato K, Amanuma M, et al. Superficial siderosis of the central nervous system caused by hemorrhagic intraventricular craniopharyngioma: case report and literature review. Neurol Med Chir (Tokyo). 2015; 55:89–89.

4. Zoia C, Cattalani A, Turpini E, et al. Haemorrhagic presentation of a craniopharyngioma in a pregnant woman. Case Rep Neurol Med. 2014; 2014:435208.

5. Miliaras G, Bostantjopoulou S, Argyropoulou M, Kyritsis A, Polyzoidis K. Superficial siderosis of the CNS: report of three cases and review of the literature. Clin Neurol Neurosurg. 2006; 108:499–502.

6. Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol. 2007; 3:54–58.

7. Glastonbury CM, Osborn AG, Salzman KL. Masses and malformations of the third ventricle: normal anatomic relationships and differential diagnoses. Radiographics. 2011; 31:1889–1905.

8. Vyas S, Prabhakar N, Tewari MK, Radotra BD, Khandelwal N. Hypothalamic glioma masquerading as craniopharyngioma. J Neurosci Rural Pract. 2013; 4:323–325.

9. Dasa JM, Rajmohan BP, Krishna B, Peethambaran , Anilkumar . Hemorrhage into craniopharyngioma as a differential diagnosis of pituitary apoplexy: a case report and literature review. Kerala Med J. 2015; 8:33–37.
10. Nishioka H, Ito H, Haraoka J, Hashimoto T, Kato Y. Repeated hemorrhage in ciliated craniopharyngioma--case report. Neurol Med Chir (Tokyo). 2000; 40:324–332.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download