Abstract
Pelvic organ prolapse (POP) is bulging of one or more of the pelvic organs into the vagina and triggered by multiple causes. It is a very common disorder, especially among older women. POP is characterized by protrusion of the presentation part visible by the naked eye, and problems with urination or bowel movements. POP can be diagnosed based on the onset of symptoms and a pelvic exam, and management options include medical and surgical treatment. Although medical treatment cannot correct the abnormal herniation of the pelvic structures, this can help alleviate symptoms. One of the disadvantages of surgical interventions is recurrence, and advances in surgical techniques have decreased recurrence rates of POP. Therefore, author will explain the gynecology and urology approach and treatment.
Pelvic organ prolapse (POP) refers to the protrusion of one or more female pelvic organs outside the pelvis through the vagina including uterus, bladder and intestines, and causes the pelvic organs to drop downward to the vaginal wall.1 In the UK, POP accounts for 20% of women waiting for major gynecological surgery and is a leading indication for hysterectomy in postmenopausal women.2 POP is divided into 3 categories, and anterior vaginal wall prolapse, or cystocele is the most common form of vaginal prolapse and a cystocele also includes a protrusion of the bladder into the vagina. Apical prolapse includes the uterus or post-hysterectomy vaginal cuff, and may involve small intestine (enterocele), bladder, or colon (sigmoidocele). Posterior vaginal wall prolapse involves a herniation of the rectum (rectocele) and may affects small or large bowel.3
POP is a major indication for gynecological surgery, but epidemiological studies on the incidence and prevalence of POP have been rarely reported.1 The accurate prevalence of POP is unknown because different classification systems have been used in diagnosis; POP rates have been assessed regardless of whether there is any symptom; and the exact number of POP patients who believe that they need medical care has not been identified. Furthermore, delayed or denied treatment accounts for a large percentage because patients do not reveal POP symptoms due to embarrassment. In the Women's Health Initiative study, POP was detected in 41% of women in the age ranges of 50 to 59 years. This included cystocele in 34%, rectocele in 19%, and uterine prolapse in 14%.4 The incidence of prolapse surgery is 4.9 cases per 1,000 women, and the peak incidence of surgery is for women aged 60 to 69 years. Almost 58% of procedures are performed in women younger than 60 years, and recurrence rate was 13% and reoperation for prolapsed was undertaken within 5 years after primary surgery.56
The risk factors for POP include vaginal child birth, advancing age, and obesity.7 Of these, vaginal child birth and obesity, in particular abdominal obesity, are profoundly related to POP. In the Oxford Family Planning study, as compared with nulliparous women, the risk of POP increased 4-fold after the first vaginal childbirth, 8-fold after the second delivery and 9-fold after the third delivery, indicating the increased risk of POP after repeated vaginal delivery.8
Advancing age is one of the leading risk factors for POP. Both incidence and prevalence of POP increase with advancing age.1 As demonstrated by a study of about 1,000 females in the U.S., the risk of POP increased by about 40% with every 10 years of age.9
Overweight (body mass index [BMI] ≥25) and obesity (BMI ≥30) are also main risk factors for POP. Overweight and obese women are at increased risk for POP compared to those with a normal weight. An analysis of 22 different studies has found that the risk of developing POP increased by about 40% to 50% in overweight and obese women compared to normal-weight women.10 On the contrary, the impact of weight loss on POP regression has been investigated and as results, weight loss does not appear to be associated with POP regression.11
POP patients having prolapsed organs often complain of a bearing-down sensation, heaviness with urination/defecation, or discomfort in the lower abdomen, and in severe cases, the uterus or bladder may even protrude out of the vagina grossly visible.3
POP patients commonly complain of a pressure sensation, a bearing-down sensation and other symptoms in the vagina or pelvis, and the association between the intensity of sensation and prolapse stage has not yet been clarified.13 The hymen seems to be an anatomical cut-off point of symptomatic prolapse. In prolapse beyond the hymen, specificity for a bearing-down sensation is 99% to 100%, but relatively low sensitivity for more severe prolapse ranges between 16% to 35% because there are asymptomatic cases.14 Symptoms are more severe in cases of bladder prolaspe, and symptoms are manifested in the leading point of prolaspe regardless of the hymen.
POP may even influence bladder or urethral function, as a result of weakening of support of the anterior vaginal wall or vaginal apex. Most patients complain of stress incontinence, and those with advanced POP beyond the hymen complain of voiding dysfunction, as a result of direct pressure on the urethra. Patients may also experience discomfort after a bowel movement, and their major complaints are constipation or tenesmus.15 Furthermore, coital function may be affected, because POP patients are more likely to avoid sexual intercourse due to fear of fecal and/or urinary incontinence during sexual activity.16
POP is typically diagnosed by a pelvic examination. Assessment is done after increasing patient's abdominal pressure whiling relaxing with subjects in the supine position. The degree of prolapse is also evaluated while the patient is standing and the vagina is examined to determine which parts of the vagina (anterior, posterior, or apical) is prolapsing. An imaging test is helpful in detecting enterocele or intussusception especially among patients having severe bulging symptoms without any signs of prolapse in a physical exam. Inquiry is an important step in diagnosing POP and identifying prolapse-related symptoms because treatment is often used for patients with symptomatic prolapse.
Management of POP is carried out when protrusion, urinary, bowel, or sexual dysfunction and other symptoms are associated with POP. Patients with asymptomatic POP usually do not require treatment. Conservative or surgical management is performed in symptomatic patients, and treatment choices depend on patient's preferences. Conservative management is appropriate for patients who are at high risk of complications and recurrence after surgical management or who refuse to undergo surgical interventions. Treatment options include insertion of pessaries, pelvic floor muscle exercises, hormone therapy and others.
A pessary is a device that is placed into the vagina to help relieve prolapse by supporting the pelvic organs and easing pressure on the bowel, and pessaries come in varying shapes and sizes. After insertion of pessary, patients need to confirm whether the pessary is in the correct position by performing various activities of daily living (sitting, standing and bending postures, Valsalva maneuver). All patients should be checked within 4 to 6 weeks after pessary insertion. A pessary requires regular care to examine new symptoms and the presence of a lesion on the vagina. In case of erosion, vaginal estrogen cream or estradiol vaginal tablets can be used. A pessary needs to be changed at regular intervals. Biopsy of erosions that persist despite intervention should be considered. The most common side-effects are discharge and a bad odor, severe complications include vesicovaginal or rectovaginal fistula, hydronephrosis and others.19 The pessary also should be sterilized routinely.
A meta-analysis of pelvic floor muscle training in about 2,300 patients suggested that muscle strengthening exercises had positive effects on improvement in prolapse stage and symptoms.20 Although a study described that systemic and topical estrogen hormone therapies can help relieve POP, data are insufficient to suppor their efficacy.
Surgical management is performed in cases of failure of conservative management and patient choice. A variety of surgical options have been introduced to choose from depending on the use of graft and a vaginal or abdominal approach (Fig. 1).21
Most patients with symptomatic POP undergo a reconstructive procedure. An obliterative procedure is an alternative for those who cannot tolerate extensive surgery, are at old ages, and no longer desire preservation of coital function. An anti-incontinence procedure is additional treatment for those stress urinary incontinence (SUI) or advanced apical prolapse. Hysterectomy can be considered a treatment option for patients with apical prolapse, in particular, in women who desire to eliminate any chance of cervical or intrauterine pathology, do not desire to get pregnant, or do not wish to preserve future fertility. The abdominal surgical route is primarily taken in patients with anterior and posterior prolapse and high risk of recurrence. Since recurrence rates of prolapse have been reported up to 30%, the risk of prolapsed recurrence should be informed to patients before performing an operation.622
Surgical mesh is the standard treatment in abdominal sacral colpopexy or hysteropexy. It has been proven that the use of surgical mesh reduces hernia recurrence rate in comparison to hernia repair without surgical mesh.23 The mesh-related side-effects are erosion, infection and others, and removal of mesh is helpful in relieving symptoms of persistent pain, coital discomfort, severe vaginal discharge and others.24
In recent years, subtotal hysterectomy, para-vaginal repair and mesh induced sacrocolpopexy have been performed via robotic-assisted surgery, and these procedures contribute to reduce recurrence (Fig. 2A).
Anterior vaginal wall prolapse is defined as the decent of the anterior vagina such that the urethra-vesical junction (a point 3 cm proximal to the external urethral meatus) or any anterior point proximal to this is less than 3 cm above the plane of the hymen. The basic principle of surgery for cystocele is to correct anatomical defects. Surgery is performed through the vagina or abdomen, and laparotomy, laparoscopic, and robotic systems can be applied.
Cystocele is classified as paravaginal defect (central, displacement), midline defect (central, distention) or transverse defect (apical) depending on whether the pubocervical fascia is separated from the vaginal cuff, separated from the uterosacral ligament, or mixed.
Lateral cystocele occurs when the pubocervical fascia and pubourethral ligament are separated from the arcus tendinosus fasciae pelvis (ATFP). Depending on the mechanism of the lateral cystocele, the aim of the surgery is to correct loose or detached pubocervical and pubourethral ligaments.
After perforating the endopelvic fascia, palpate with finger to identify the ATFP. The use of an appropriate retractor is useful because the surgical field is very deep. Place 5 to 7 interrupted nonabsorbable sutures on both sides at approximately 1 cm intervals with the bladder retracted medially. Recently, a procedure using a trocar-guided transvaginal mesh has been generally performed to reliably correct and strengthen the defect, and various meshes such as acellular collagen biomesh have been used.25 After the tie of the sutures is completed, a cystoscopy is performed to check the efflux from the ureteral orifice and bladder damage. The success rate of transvaginal paravaginal repair has been reported from 82.5% to 98%.26
This approach is recommended when performing Burch operation or total abdominal hysterectomy. The detached pubocervical fascia is sutured to ATFP using nonabsorbable sutures at 1 cm intervals from the ischial spine to the pubic bone. Reported success rates are 85% to 98%.2728 Recently, retropubic paravaginal repair has been performed in most cases using laparoscopy and cure rate is similar to open surgery.29 Complications include intraoperative bleeding, lower extremity neuropathy, vaginal abscess, and ureteral obstruction.
Central cystocele is caused by the inability to support the pubocervical fascia in the midline of the anterior vaginal wall. The aim of surgical correction is to approximate an elongated or separated pubocervical fascia.
This procedure was first performed by Kelly in 1913, and many modified procedures have since been introduced. However, it is a basic principle to approximate weakened pubocervical fascia with interrupted suture using 2–0 delayed absorbable sutures. Cure rates were reported up to 97%.30 However, recent reports suggest that the recurrence rate of cystocele is 70% after anterior colporrhaphy.31 If there is a severe weakening of the pubocervical fascia, place synthetic meshes or a biological graft under the repaired pubocervical fascia. Sand et al.32 compared the 1 year follow-up of the anterior colporrhaphy with and without the polyglactin mesh placed under the pubocervical fascia. The failure rate of randomized female patients who underwent surgery with mesh was 25%, whereas female patients who underwent anterior colporrhaphy alone had a failure rate of 43%.32 According to another recent report, the transvaginal mesh repair group showed a higher cure rate than the traditional anterior colporrhaphy group at 1 year postoperatively (60.8% vs. 34.5%), but the incidence of bladder perforation and new stress incontinence was higher.33 This procedure is not recommended for correction of lateral defect or stress incontinence. Although intraoperative bleeding is not common, complications such as postoperative hematoma formation, bladder and urethral injury, ureteral injury and obstruction, urinary tract infection, and voiding dysfunction are possible complications.
This procedure is not recommended for patients with central cystocele alone. Intra-abdominal anterior repair may be performed in patients with mild central cystocele requiring abdominal surgery such as hysterectomy. After dissection of the bladder and vagina, wedge resection of the redundant vaginal wall is performed. After this, interrupted or running suture is performed and corrected. Lovatsis and Drutz34 reported a success rate of intra-abdominal anterior repair of grade 1 cystocele to 89%. However, the recurrence rate was 30% at 2 years and 61% at 5 years.
Most cystoceles occur as a mixture of lateral and central defects. The purpose of the surgery is to correct the anatomical deficits that cause.
Anterior colporrhaphy with paravaginal repair can be performed in the manner described above. However, there is a difficulty in performing paravaginal repair after anterior colporrhaphy. This is because the vector force of the other direction acts on the pubocervical fascia. One facing inward and the other facing outward. The different actions of these vector forces are one of the reasons for producing synthetic mesh or biological graft material kits. Currently, despite the controversy over synthetic mesh, some urologists and gynecologists continue to implement this methodology for additional advantages and convenience.35 Rodríguez et al.36 reported a cure rate of grade 4 cystocele as 84%.
The transabdominal approach is not recommended for patients with only lateral and central defect cystocele without any other associated disease requiring intra-abdominal surgery. Anterior repair through the abdomen is also limited in the success of treatment due to the difficulty of approximation of the pubocervical fascia.34 However, in women with apparent SUI associated with advanced prolapse, placement of midurethral mesh sling through the abdomen or Burch procedure results in a higher continence rate than paravaginal repair or suburethral plication alone (Fig. 2B).
POP is a common disorder among older women and requires appropriate management. However, the diagnosis can be delayed due to a variety of reasons. In primary care, lifestyle advices in daily living include weight loss, avoiding of heavy lifting, management of constipation and pelvic floor muscle training. Patients complaining of persistent symptoms and discomfort should be offered a wide range of treatment options available for improved quality of life.
Figures and Tables
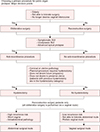 | Fig. 1Choosing a primary procedure for pelvic organ prolapse: major decision points. SUI: stress urinary incontinence. [Reprinted from “Pelvic organ prolapse in women: Choosing a primary surgical procedure”, by Jelovsek E, 2018, Waltham, MA, UpToDate, Inc., Available from: https://www.uptodate.com/contents/pelvic-organ-prolapse-inwomen-choosing-a-primary-surgicalprocedure?search=Pelvic%20organ%20prolapse%20in%20women:%20Choosing%20a%20primary%20surgical%20procedure&source=search_result&selectedTitle=1~150. Copyright 2018 by UpToDate, Inc. Reprinted with permission]. |
References
1. Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998; 25:723–746.

2. Lepine LA, Hillis SD, Marchbanks PA, Koonin LM, Morrow B, Kieke BA, et al. Hysterectomy surveillance-United States, 1980-1993. MMWR CDC Surveill Summ. 1997; 46:1–15.
4. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002; 186:1160–1166.

5. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997; 89:501–506.

6. Clark AL, Gregory T, Smith VJ, Edwards R. Epidemiologic evaluation of reoperation for surgically treated pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2003; 189:1261–1267.

7. Greer WJ, Richter HE, Bartolucci AA, Burgio KL. Obesity and pelvic floor disorders: a systematic review. Obstet Gynecol. 2008; 112:341–349.
8. Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997; 104:579–585.

9. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol. 2003; 189:372–377.

10. Giri A, Hartmann KE, Hellwege JN, Velez Edwards DR, Edwards TL. Obesity and pelvic organ prolapse: a systematic review and meta-analysis of observational studies. Am J Obstet Gynecol. 2017; 217:11–26.

11. Kudish BI, Iglesia CB, Sokol RJ, Cochrane B, Richter HE, Larson J, et al. Effect of weight change on natural history of pelvic organ prolapse. Obstet Gynecol. 2009; 113:81–88.

12. Jørgensen S, Hein HO, Gyntelberg F. Heavy lifting at work and risk of genital prolapse and herniated lumbar disc in assistant nurses. Occup Med (Lond). 1994; 44:47–49.

13. Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001; 185:1332–1337.

14. Bradley CS, Nygaard IE. Vaginal wall descensus and pelvic floor symptoms in older women. Obstet Gynecol. 2005; 106:759–766.

15. Whitcomb EL, Lukacz ES, Lawrence JM, Nager CW, Luber KM. Prevalence and degree of bother from pelvic floor disorders in obese women. Int Urogynecol J Pelvic Floor Dysfunct. 2009; 20:289–294.

16. Novi JM, Jeronis S, Morgan MA, Arya LA. Sexual function in women with pelvic organ prolapse compared to women without pelvic organ prolapse. J Urol. 2005; 173:1669–1672.

17. Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996; 175:10–17.

18. Steele A, Mallipeddi P, Welgoss J, Soled S, Kohli N, Karram M. Teaching the pelvic organ prolapse quantitation system. Am J Obstet Gynecol. 1998; 179:1458–1463.

19. Abdulaziz M, Stothers L, Lazare D, Macnab A. An integrative review and severity classification of complications related to pessary use in the treatment of female pelvic organ prolapse. Can Urol Assoc J. 2015; 9:E400–E406.

20. Li C, Gong Y, Wang B. The efficacy of pelvic floor muscle training for pelvic organ prolapse: a systematic review and meta-analysis. Int Urogynecol J. 2016; 27:981–992.

21. Jelovsek E. Pelvic organ prolapse in women: Choosing a primary surgical procedure. Waltham, MA: UpTo-Date, Inc;2018. Cited by 2018 Nov 15. Available from: https://www.uptodate.com/contents/pelvic-organprolapse-in-women-choosing-a-primary-surgicalprocedure?search=Pelvic%20organ%20prolapse%20in%20women:%20Choosing%20a%20primary%20surgical%20procedure&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
22. Gilchrist AS, Campbell W, Steele H, Brazell H, Foote J, Swift S. Outcomes of observation as therapy for pelvic organ prolapse: a study in the natural history of pelvic organ prolapse. Neurourol Urodyn. 2013; 32:383–386.

23. De La Cruz JF, Myers EM, Geller EJ. Vaginal versus robotic hysterectomy and concomitant pelvic support surgery: a comparison of postoperative vaginal length and sexual function. J Minim Invasive Gynecol. 2014; 21:1010–1014.

24. Fairchild PS, Kamdar NS, Berger MB, Morgan DM. Rates of colpopexy and colporrhaphy at the time of hysterectomy for prolapse. Am J Obstet Gynecol. 2016; 214:262.

25. Citgez S, Demirkesen O, Ozdemir F, Gevher F, Demirdag C, Onal B, et al. Transvaginal repair using acellular collagen biomesh for the treatment of anterior prolapse. Urol J. 2014; 11:1271–1277.
26. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001; 185:1360–1366.

27. Bruce RG, El-Galley RE, Galloway NT. Paravaginal defect repair in the treatment of female stress urinary incontinence and cystocele. Urology. 1999; 54:647–651.

28. Scotti RJ, Garely AD, Greston WM, Flora RF, Olson TR. Paravaginal repair of lateral vaginal wall defects by fixation to the ischial periosteum and obturator membrane. Am J Obstet Gynecol. 1998; 179:1436–1445.

29. Miklos JR, Kohli N. Laparoscopic paravaginal repair plus burch colposuspension: review and descriptive technique. Urology. 2000; 56:64–69.

30. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994; 171:1518–1526.

31. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001; 185:1299–1304.

32. Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001; 184:1357–1362.

33. Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C. Anterior colporrhaphy versus transvaginal mesh for pelvicorgan prolapse. N Engl J Med. 2011; 364:1826–1836.

34. Lovatsis D, Drutz HP. Is transabdominal repair of mild to moderate cystocele necessary for correction of prolapse during a modified Burch procedure? Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12:193–198.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download