This article has been corrected. See "Correction: Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells" in Volume 49 on page 93.
Abstract
Minor ginsenosides Rh1 and Rg2 were isolated from Korean red ginseng and reported to have various biological effects on anti-inflammatory and anti-stress activities. However, the effects of Rh1 and Rg2 on antioxidant activity and their regulatory effects on the antioxidant enzymes have not been studied. Since oxidative stress is one of the major toxic inflammatory responses stimulated by lipopolysaccharides (LPS), the present study investigated the role of minor ginsenosides Rh1 and Rg2 on antioxidant effects in LPS-treated RAW 264.7 cells. In this study, we found that treatment with ginsenosides Rh1 and Rg2 strongly inhibited LPS-stimulated intracellular ROS production in cells. Luciferase assay showed that treatment with LPS reduced antioxidant response element (ARE) encoding the pARE-luc promoter activity, while ginsenosides inhibited the pARE-luc promoter activity. Moreover, ginsenosides Rh1 and Rg2 exhibited anti-oxidative activity in LPS-induced cells by upregulating antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase. Our results suggest that minor ginsenosides Rh1 and Rg2 may be potential bio-active compounds for antioxidative effects by inhibiting the generation of ROS in RAW 264.7 cells.
Lipopolysaccharides (LPS) known as lipoglycans and endotoxin, is an important component of the Gram-negative bacteria outer membrane (1). It is involved in a variety of immune responses by binding CD14/TLR4/MD2 receptor complexes in many cell types including monocytes, dendritic cells, macrophages, and B cells (23). It has been shown that LPS stimulates innate immunity and prolonged exposure to high doses of LPS induces oxidative stress caused by production of reactive oxygen species (ROS) (34). Since ROS molecules are highly reactive low molecular weight lipophilic species, the ROS molecules can easily penetrate cell walls and induce cell damage (35). Therefore, high level of ROS production not only induces cell damage but also stimulates downstream signaling pathways and expends the immune responses (46).
The ROS, such as superoxide anion (O2−), hydroxyl (OH−), perosyl (ROO−) or hydrogen peroxide (H2O2), are mainly produced in the mitochondria during cellular metabolism through the respiratory chain (78). These ROS are highly unstable and reactive, so they can easily react with damaged cell compartments such as lipids, proteins, and DNA (9). On the other hand, the cells have a defense system with antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) against harmful ROS (10). For example, SOD converts O2− anions to H2O2 and H2O2 is rapidly removed by detoxifying enzymes such as GPx. In addition, CAT decomposes and removes H2O2 into water and oxygen. Therefore, antioxidant enzymerich bioactive compounds could protect the body from chronic diseases caused by ROS.
Recent studies have suggested that induction of detoxifying enzyme activity is regulated by transcriptional levels in the cis-acting element located within the promoters of the detoxifying enzyme genes called the antioxidant-responsive element (ARE) (11). This element is also found in the gene promotors encoding cellular antioxidant enzymes, such as haem oxygenase-1 (12) and glutathione-S-transferase (13) and is activated by various oxidative stress. Therefore, binding to DNA site of ARE is one of the major mechanisms in cellular defense from ROS, resulting in target gene expression associated with detoxification and elimination of ROS (14).
Ginseng is an herb that is famous in eastern Asian countries and has recently been used widely in Western countries. One of the components of ginseng, ginsenoside, has been reported to have various biological effects including anti-tumor, anti-inflammation and anti-stress activities (151617). The ginsenosides were classified into two groups, protopanaxadiol (PPD) and protopanaxatriol (PPT) (18). The difference between PPD and PPT types is that PPD types have carboxyl group at the C-6 position, but PPT types do not. The PPD group includes ginsenosides of Rb1, Rb2, Rc, Rd, Rg3 and Rh2, and the PPT group includes ginsenosides of Re, Rf, Rg1, Rg2 and Rh1. Among the PPT type ginsenosides, Rh1 and Rg2 can be classified as minor ginsenosides, which are deglycosylated from the major ginsenosides. It has been reported that ginsenosides Rh1 and Rg2 protect the rheological functions of erythrocytes against oxidative stress (19). However, the effects of Rh1 and Rg2 on antioxidant activity and their regulatory effects on the antioxidant enzymes have not been studied. Therefore, we investigated the effect of ginsenosides Rh1 and Rg2 on antioxidant activity and antioxidant-related gene expression in LPS-induced RAW 264.7 cells.
3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, #M6494) was purchased from Invitrogen (Waltham, MA, USA). Dulbecco's modified Eagle's medium (DMEM, #11963-092), and fetal bovine serum (FBS, #10082147) were purchased from Gibco (Waltham, MA, USA). 1, 1-Diphenyl-2picrylhydrazyl (DPPH, #D9132), lipopolysaccharide (LPS, E. coli, O111:B4, #L2630) and 2′-7′-Dichlorofluorescin diacetate (DCF-DA, #D6883) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phosphate buffered saline (PBS, #EBA-1105) and reveres transcription 5X master mix (#EBT-1511) were purchased from ELPIS-BIOTECH (Daejeon, Korea). Tri-RNA reagent (#FATRR-001) was purchased from Favorgen (PingTung, China). Ginsenosides Rh1 and Rg2 (purity 99%) were purchased from Ace EMzyme (Anseong-si, Korea). Rh1 and Rg2 is mixed in a 1:1 ratio.
The murine macrophage RAW 264.7 cell was purchased from Korea Cell Line Bank (KCLB, #40071, Seoul, Korea) and maintained in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin at 37℃ in a humidified atmosphere with 5% CO2 (HERAcell 150i, Thermo Electron Corp., Waltham, MA, USA). For experiments, RAW 264.7 cells were seeded in 96-well plates at the density of 1 × 104 cells/well. After serum starvation for 24 h, cells were treated with FBS-free medium containing various concentrations (0, 1, 5, 10, 25 or 50 µg/ml) of ginsenosides Rh1 and Rg2 followed by treatment with LPS (500 ng/ml) for indicated time in each Figure legend.
The DPPH radical scavenging activity of the Rh1 and Rg2 was determined according to the previous report (20). Briefly, samples at various concentrations were added to 5 µl of DPPH (2.5 mg/ml) in 100% ethanol. The mixture was mixed and allowed to stand for 30 mins at 25℃ dark conditions. The absorbance was measured at 517 nm using microplate reader (TECAN, Männedorf, Switzerland). Vitamin C (Vit. C) was used as a positive control. The free radical-scavenging capacity was expressed by relative percentage of control.
The ABTS [2,2′-azino-bis(3-ethlybenzthizoline-6-sulphonic acid)] assay was performed to scavenge the ABTS radical cation in comparison to the compounds. The radical cation was prepared by mixing 7 mM ABTS with 2.4 mM potassium persulfate (1:1 v/v) and leaving the mixture for 24 h until the reaction was completed and the absorbance was stable. The ABTS radical solution was diluted with PBS and absorbance of 0.7 (±0.02) at 732 nm. The assay was conducted with diluted ABTS radical solution mixed with samples, and the measurements were taken at 732 nm after 30 mins. The anti-oxidative activity of the samples was calculated by determining the decrease in absorbance.
The mitochondrial-dependent reduction of MTT to formazan was used to measure cell respiration as an indicator of cell viability (21). Briefly, RAW 264.7 cells were seeded in 96-well plates at the density of 1 × 104 cells/well. After 24 h of incubation, fresh FBS free medium were replaced for 24 h. The adhered cells were treated with FBS-free medium containing various concentrations (0, 1, 5, 10, 25 or 50 µg/ml) of the compound. After 24 h, MTT (12 mM) was prepared in FBS-free DMEM media to give a total volume of 100 µl in each well and the cells were incubated for 2 h at 37℃ and 5% CO2. The medium was the removed and the formazan precipitate was solubilized in DMSO. The absorbance was measured at 540 nm on a microplate reader (TECAN).
Intracellular ROS levels were measured by detecting the fluorescent intensity of cells with 2′,7′-dichlorodihydrofluoresceindiacetate (DCFH-DA) (22). RAW 264.7 cells were seeded in 96-well plate at the 1 × 104 cells/well. Cells were then starved for 24 h in the serum free DMEM. Then the cells were treated with various concentrations of compounds. After 1 h, the cells were stimulated with or without medium containing LPS (500 ng/ml) at 37℃ for 24 h. Cells were washed with PBS and incubated at 37℃ for 30 min (in dark) with the probe at a final concentration of 10 µM DCFH-DA. Cells were washed with PBS and the fluorescence intensity of DCF was measured by using the fluorescence microplate reader with an excitation wavelength of 485 nm and an emission wavelength of 530 nm (TECAN).
The DNA construct of antioxidant response element (ARE) encoding pARE-luc plasmid was obtained by Promega (Madison, WI, USA). The DNA construct contains four copies of ARE (5′-TGACnnnGCA-3′) that drives transcription of the luciferase reporter gene luc2P (Photinus pyralis). RAW 264.7 cells were plated on 12-well plates at 5 × 104 cells/well. For a luciferase assay, cells were transfected in Opti-MEM (#31985-088, Invitrogen) with lipofectamine mixture containing the pARE-luc reporter plasmid along with pRL-CMV for 18 h. Cells were pretreated with 10 µg/ml of Rh1 and Rg2 compounds for 1 h followed by treatment with 500 ng/ml of LPS for 6 h. The cells were collected after treatment, and the luciferase activity was assayed with the dual luciferase kit (E1960, Promega) using a TD-20/20 luminometer (Turner Designs, San Jose, CA, USA). Since pRL-tk contains the Renilla luciferase gene, the expression and transfection efficiencies were normalized with the Renilla luciferase activity. Transfections were performed in triplicate, and each experiment was repeated at least three times.
Quantitative RT-PCR (qRT-PCR) assay was used to analyze the mRNA expression of anti-oxidative enzymes including Cu/ZnSOD, MnSOD, catalase, or GPx according to the previous report (23). Total RNA was isolated from cells using Tri-RNA reagent according to the manufacturer's instructions. The 1 µg of total RNA in each group was reverse transcribed into single-stranded cDNA using the reverse transcription 5X master mix. The mRNA expression of the genes was quantified with iQ™ SYBR green supermix (#170-8882, Bio-Rad, Hercules, CA, USA) using a CFX Connect™ (Bio-Rad). The relative gene expression was calculated using the 2−ΔΔct method, and GAPDH was used for normalization. All primer sequences used in qRT-PCR experiments are listed in Table 1.
Statistical analysis was performed using GraphPad Prism 5 (version 5.02, GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison was performed. A p value <0.05 was considered significant. All experiments were expressed as the mean ± SEM and were performed independently at least 3 times.
Cell viability tests were performed using MTT assay to determine the experimental concentration of the compounds. The serum starved RAW 264.7 cells were treated with various concentrations of Rh1 and Rg2 compounds (1, 5, 10, 20, 40, 60, 80 and 100 µg/ml) for 24 h. The results showed that there was no cytotoxic effect on RAW 264.7 cells at the tested concentrations (Fig. 1). Therefore, ginsenosides Rh1 and Rg2 concentrations of 1 to 50 µg/ml were selected in subsequent experiments.
The radical scavenging capacity of the compounds were determined by DPPH and ABTS assay. Various concentrations of Rh1 and Rg2 compounds were mixed with the free radical of DPPH solution and then residual free radical DPPH was measured at 518 nm using a plate reader. Surprisingly, the scavenging effect of DPPH radical was not shown by treatment with any concentrations of Rh1 and Rg2 compounds (Fig. 2A). The in vitro radical scavenging effect of the Rh1 and Rg2 compounds was confirmed with ABTS analysis. Consistent with the data of the DPPH assay, the reduction of ABTS radical was also not observed by treatment of the Rh1 and Rg2 compounds (Fig. 2B).
To investigate the effect of Rh1 and Rg2 on intracellular ROS production, RAW 264.7 cells were treated with various concentrations of the compounds for 24 h followed by treatment with serum-free medium containing H2DCF-DA. Interestingly, Rh1 and Rg2 compound treatment inhibits intracellular ROS production in a dose-dependent manner in unstimulated RAW 264.7 cells (Fig. 3A). When the cells were treated with 500 ng/ml LPS for 2 h, the ROS levels were strongly increased by more than 8-fold compared to the unstimulated condition (Fig. 3B). When cells were co-treated with Rh1 and Rg2 compounds following LPS treatment, LPS-induced ROS levels were significantly suppressed at the concentrations of 10, 25, and 50 µg/ml (Fig. 3B). Vitamin C (Vit C) was used as a positive control.
To investigate how ginsenosides Rh1 and Rg2 exert antioxidative effect by eliminating intracellular ROS production, pARE-luc was used to confirm that ginsenosides Rh1 and Rg2 regulates cis-acting element placed in the promoters of the detoxifying enzyme genes. For experiment, cells were transfected with pARE-luc to analyze the activity of the firefly luciferase reporter containing sequences of the anti-oxidant response elements and cotransfected with pRL-CMV to normalized reporter activity with the Renilla luciferase expression. Treatment with 500 ng/ml LPS significantly decreased ARE transcriptional activity whereas treatment with Rh1 and Rg2 reversed LPS-induced inhibition (Fig. 4).
To confirm how Rh1 and Rg2 compounds inhibit LPS-induced intracellular ROS production, mRNA levels of anti-oxidant enzymes, such as Cu/Zn SOD, Mn-SOD, CAT, and GPx were examined using qRT-PCR analysis. As shown in Fig. 5, treatment with Rh1 and Rg2 compounds significantly increases the mRNA expression of Cu/Zn SOD, Mn-SOD, CAT, and GPx compared to that in the LPS-induced control group at 10, 25, and 50 µg/ml.
Ginsenosides Rh1 and Rg2 have been reported to possess various biological activates, including anti-inflammatory, anti-allergic and antioxidant properties (151617). In addition, recent report shows that ginsenosides Rh1 and Rg2 have an anti-rheological function of erythrocytes against oxidative stress (19). However, there was lack of information on the regulatory effect of ginsenosides Rh1 and Rg2 on antioxidant activity and antioxidant enzymes. In this study, we aimed to investigate the effect of Rh1 and Rg2 on antioxidant activity and antioxidant-related gene expression in LPS-stimulated RAW 264.7 cells.
Many studies have shown the antioxidant effects of bio-active compounds using DPPH and ABTS assay (2425). Both DPPH and ABTS are free radicals and if the compound itself is effective as an antioxidant, the compound can remove free radicals from the tube reaction. Ginsenosides Rh1 and Rg2 did not show in vitro radical scavenging effects on DPPH and ABTS radicals (Fig. 2). Instead, these bio-active compounds showed an inhibitory effect on intracellular ROS production in RAW 264.7 cells (Fig. 3). Cells treated with high doses of LPS (500 ng/ml) for 2 h strongly enhanced intracellular ROS levels, whereas treatment with Rh1 and Rg2 significantly inhibited LPS effect. We therefore investigated how ginsenosides Rh1 and Rg2 inhibited LPS-stimulated intracellular ROS production.
Previous studies have suggested that antioxidant defensive genes contribute to the response to cellular oxidative stress along with induction of genes encoding catalytic and modifier subunits, which are important for glutathione synthesis (26). These antioxidant defensive genes are up-regulated through the ARE located within the promoters of their respective genes, such as HO-1 (12) or glutathione-S-transferase (13). Interestingly, treatment with LPS reduced ARE reporter activity, whereas treatment with ginsenosides Rh1 and Rg2 reversed LPS-induced inhibition (Fig. 4). Therefore, it is possible that ginsenosides Rh1 and Rg2 have antioxidant effects in RAW-264.7 cells by the upregulation of ARE reporter activity. Recent report has demonstrated that the enhancement of HO-1 expression inhibits LPS-induced macrophage activation through upregulation of the ARE binding site of the HO-1 promoter and interaction with nuclear factor erythroid-2-related factor (Nrf-2) (27). To understand the mechanism of ginsenosides Rh1 and Rg2 having an antioxidative effect through the regulation of ARE-luc activity, investigating the effects of ginsenosides Rh1 and Rg2 on HO-1 expression, Nrf-2 transcriptional activity, and interaction between HO-1 and Nrf-2 will be good targets for future study.
It has been well established that SODs, such as Cu/Zn-SOD and Mn-SOD, are the first line of defense for superoxide free radicals (28). SODs have distinct subcellular localization depending on the metal ion cofactors (29). For example, Cu/Zn-SOD is mainly localized in the cytoplasm and Mn-SOD is localized in the mitochondria (29). Figs. 5A and B show that treatment with LPS inhibits all mRNA levels of SOD, but treatment with ginsenosides Rh1 and Rg2 results in dose-dependent increase in the mRNA levels of SOD. It suggests that ginsenosides Rh1 and Rg2 strongly upregulate expression of SOD in cytoplasm and mitochondria. In addition to SOD, catalase and GPx were found in many subcellular components including peroxisomes and cytoplasm, and mitochondria and nucleus, respectively. Interestingly, Rh1 and Rg2 also reversed these antioxidant enzymes from LPS-induced inhibition (Figs. 5C and C). Together, our current study found inhibitory effect of ginsenosides Rh1 and Rg2 on intracellular ROS production through regulation of pARE-luc promoter activity and upregulation of antioxidant enzyme expressions. It suggests that ginsenosides Rh1 and Rg2 would be good natural agents for treating various human diseases caused by oxidative stress with antioxidant effect.
Figures and Tables
 | Figure 1Effect of ginsenosides Rh1 and Rg2 on cell viability. RAW 264.7 cells were treated with indicated various concentrations of Rh1 and Rg2 compounds for 24 h. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are the mean ± SEM of experiments in triplicate (n=3). |
 | Figure 2Antioxidant activities of ginsenosides Rh1 and Rg2. (A) DPPH radical scavenging activity of various concentration of ginsenosides Rh1 and Rg2. (B) ABTS radical scavenging activity of various concentration of ginsenosides Rh1 and Rg2. White bar, control; black bar, ginsenoside Rh1 and Rg2; grey bar, Vit.C (positive control). Data are the mean ± SEM of experiments in triplicate (n=3). ***p<0.001 compared with no treated sample. |
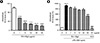 | Figure 3The effect of ginsenosides Rh1 and Rg2 on intracellular ROS production. (A) RAW 264.7 cells were treated with various concentrations of the compounds for 24 h. (B) Cells were preincubated with indicated concentrations of the compounds for 24 h and then activated with 500 ng/ml LPS for 2 h. Serum-free medium containing H2DCFDA was added to cells for 30 min. 2,7-Dichlorofluorescein fluorescence was evaluated using fluorescence microplate reader with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Data are the mean ± SEM of experiments in triplicate (n=3). ***p<0.001 compared with no treated sample. ###p<0.001 compared with LPS alone. |
 | Figure 4Effect of ginsenosides Rh1 and Rg2 on ARE promoter activity. RAW264.7 cells were transfected with ARE-luc reporter and pRL-CMV for 18 h. pRL-CMV-renilla was transfected for the internal normalization. After transfection, cells were pretreated with or without ginsenosides Rh1 and Rg2 for 1 h followed by treatment with LPS 500 ng/ml for 6 h. Cells were lysed using passive lysis buffer and luciferase activities were measured with a dual-luciferase system. Open bar, no treatment; black bar, LPS 500 ng/ml. treatment; grey bar, pretreatment with Rh1 and Rg2 followed by treatment with LPS 500 ng/ml. The relative % of the control light emission was expressed as mean ± SEM of two experiments. *p<0.05 compared with no treatment control. #p<0.05 compared with LPS alone. |
 | Figure 5Effect of ginsenosides Rh1 and Rg2 on the expression of anti-oxidant genes. (A) Cu/Zn SOD; (B) Mn SOD; (C) catalase (CAT); (D) Glutathione peroxidase (GPx) mRNA level in RAW 264.7 cells. Cells were treated with various concentrations of the compounds for 1 h followed by treatment with LPS for 6 h. Data are the mean ± SEM of experiments in triplicate (n=3). **p<0.01 compared with no treatment control. #p<0.05, ##p<0.01 and ###p<0.001 compared with LPS alone. |
ACKNOWLEDGMENT
This research was supported by SMtech Development Program (#2018-0006-01) and Research Fund of Chungnam National University (#2017-1794-01).
References
1. Baek N, Sim S, Heo KS. LPS-stimulated macrophage activation affects endothelial dysfunction. J Bacteriol Virol. 2018; 48:23–30.

2. Bang S, Lee C, Ryu J, Li W, Koh YS, Jeon JH, et al. Simultaneous determination of the bioactive compounds from Sparassis crispa (Wulf.) by HPLC-DAD and their inhibitory effects on LPS-stimulated cytokine production in bone marrow-derived dendritic cell. Arch Pharm Res. 2018; 41:823–829.

3. Choi JS, Islam MN, Ali MY, Kim YM, Park HJ, Sohn HS, et al. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and anti-inflammatory activities. Arch Pharm Res. 2014; 37:1354–1363.

4. Chen Y, Zhou Z, Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front Physiol. 2018; 9:1487.

5. Zhu X, Wang K, Zhou F, Zhu L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca(2+)/CaMKII-dependent activation of AMPK. Arch Pharm Res. 2018; 41:1009–1018.

6. Vitkov L, Hannig M, Minnich B, Herrmann M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity. 2018; 51:304–309.

7. Bombaca ACS, Viana PG, Santos ACC, Silva TL, Rodrigues ABM, Guimarães ACR, et al. Mitochondrial disfunction and ROS production are essential for anti-Trypanosoma cruzi activity of beta-lapachone-derived naphthoimidazoles. Free Radic Biol Med. 2018; 130:408–418.
8. Yu JW, Lee MS. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016; 39:1503–1518.

10. Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013; 65:1174–1194.

11. Erickson AM, Nevarea Z, Gipp JJ, Mulcahy RT. Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene. J Biol Chem. 2002; 277:30730–30737.

12. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078.

13. Nguyen T, Pickett CB. Regulation of rat glutathione S-transferase Ya subunit gene expression. DNA-protein interaction at the antioxidant responsive element. J Biol Chem. 1992; 267:13535–13539.

14. Bahia PK, Rattray M, Williams RJ. Dietary flavonoid (−)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J Neurochem. 2008; 106:2194–2204.
15. Song BK, Kim KM, Choi KD, Im WT. Production of the Rare Ginsenoside Rh2-MIX (20(S)-Rh2, 20(R)-Rh2, Rk2, and Rh3) by Enzymatic Conversion Combined with Acid Treatment and Evaluation of Its Anti-Cancer Activity. J Microbiol Biotechnol. 2017; 27:1233–1241.

16. Cui CH, Kim DJ, Jung SC, Kim SC, Im WT. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming beta-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules. 2017; 22:pii: E844.
17. Baatar D, Siddiqi MZ, Im WT, Ul Khaliq N, Hwang SG. Anti-Inflammatory Effect of Ginsenoside Rh2-Mix on Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage Cells. J Med Food. 2018; 21:951–960.

18. Jung SC, Kim W, Park SC, Jeong J, Park MK, Lim S, et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014; 55:2177–2188.

19. Samukawa K, Suzuki Y, Ohkubo N, Aoto M, Sakanaka M, Mitsuda N. Protective effect of ginsenosides Rg(2) and Rh(1) on oxidation-induced impairment of erythrocyte membrane properties. Biorheology. 2008; 45:689–700.

20. Jang H, Lee JW, Lee C, Jin Q, Lee MK, Lee CK, et al. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch Pharm Res. 2016; 39:1232–1236.

21. Park HS, Quan KT, Han JH, Jung SH, Lee DH, Jo E, et al. Rubiarbonone C inhibits platelet-derived growth factor-induced proliferation and migration of vascular smooth muscle cells through the focal adhesion kinase, MAPK and STAT3 Tyr(705) signalling pathways. Br J Pharmacol. 2017; 174:4140–4154.

22. Park HS, Han JH, Jung SH, Lee DH, Heo KS, Myung CS. Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells. Korean J Physiol Pharmacol. 2018; 22:349–360.

23. Heo KS, Le NT, Cushman HJ, Giancursio CJ, Chang E, Woo CH, et al. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest. 2015; 125:1299–1310.

24. Ravanfar SA, Karimi E, Mehrabanjoubani P, Ebrahimi M. Enhancement of phenolic and flavonoids compounds, antioxidant and cytotoxic effects in regenerated red cabbage by application of Zeatin. Nat Prod Res. 2018; 1–5.

25. Hoyos-Arbeláez J, Blandón-Naranjo L, Vázquez M, Contreras-Calderón J. Antioxidant capacity of mango fruit (Mangifera indica). An electrochemical study as an approach to the spectrophotometric methods. Food Chem. 2018; 266:435–440.

26. Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993; 268:19675–19680.

27. Cho RL, Yang CC, Tseng HC, Hsiao LD, Lin CC, Yang CM. Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARgamma attenuates LPS-mediated lung inflammation. Br J Pharmacol. 2018; 175:3928–3946.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download