This article has been
cited by other articles in ScienceCentral.
Dear Editor:
A 75-year-old woman presented with generalized erythematous pruritic patches and papules on the face, neck and both extremities which occurred 2 months ago. The patient has developed Idiopathic pulmonary fibrosis (IPF) for 10 years and she was treated with pirfenidone 5 months ago with good tolerability. No adverse effect was reported during the first 3 months of administration, and the dose of pirfenidone was gradually increased from 600 mg/day to 1,200 mg/day for the symptom control. Skin rash initially developed in the sun exposed areas, but gradually spread to the whole body. Punch biopsy was performed on the dorsum of right hand (
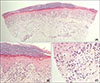
Fig. 1). We received the patient's consent form about publishing all photographic materials. Histopathology revealed lichenoid interface dermatitis, focal parakeratosis, and necrotic keratinocytes, which was consistent with lichenoid drug eruption (
Fig. 2). The patient was initially treated with oral and topical steroid, but oral steroid was discontinued due to recurrent infection. Respiratory physician reduced the dose of pirfenidone to 600 mg/day. After the dose reduction, symptoms have been controlled by topical steroids.
IPF is a progressive, fibrotic lung disease with poor prognosis. Median survival is 3~5 years without effective therapy
1. Pirfenidone is an oral antifibrotic agent which inhibits tumor necrosis factor-α and transforming growth factor-β with therapeutic effect for IPF. The primary treatment-related adverse events associated with pirfenidone are gastrointestinal upset, skin eruption. The skin eruption associated with pirfenidone has been reported in several cases related to photosensitivity, but no lichenoid drug eruption has been reported
2.
Lichenoid drug eruption is a relatively rare form that accounts for less than 1% of drug related rash. Clinical and histopathologic findings are similar to those of idiopathic lichen planus, and there is no clear distinction between them. Lichenoid drug eruption occurs symmetrically in a wide area of the trunk, mucosa and nail involvement are rare, and Wickham stria is not observed. Histologically eosinophils and plasma cells are infiltrated upper dermis
3.
Drug eruption is generally known as the immunological mechanism associated with T cell response. However, pirfenidone seems to be involved in other mechanisms because the symptoms worsen due to the increased dose. First, there is a possibility that the occurrence of reactive oxygen species (ROS) due to phototoxic damage. In murine, as the dose of pirfenidone increased, the level of ROS increased during sun exposure, and it was possible that the T cell was activated by a mechanism associated with an increase in the incidence of ROS
4. Second, there is a possibility that pirfenidone is associated with the inhibition of TNF-α. There are many reports of the lichenoid disease when TNF-α inhibitors are used. There is evidence that keratinocyte antigen expression and cytokine production may be an early event of lesion formation by keratinocyte apoptosis induced by antigen-specific CD8+ cytotoxic T cells
5. Further studies are needed to determine the exact pathogenesis.
Herein, we describe a patient with IPF who presented with lichenoid drug eruption after initiation of pirfenidone therapy. Although pirfenidone is a key drug for the treatment of idiopathic pulmonary fibrosis, clinicians should be aware of the high prevalence of pirfenidone related drug eruption.
Figures and Tables
Fig. 1
Symmetrically distributed, multiple erythematous patches and papules with focal desquamation on the whole body.
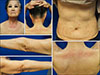
Fig. 2
(A) Hyperkeratosis and upper-dermal cellular infiltration obscures the epidermo-dermal junction. In mid to deep dermis, inflammatory cells infiltrate around vessels (H&E, ×40). (B, C) Necrotizing keratinocytes in the epidermis. Note the melanophages and lymphocytes in the cellular infiltrates (H&E: B, ×200; C, ×400).






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download