Abstract
Cognitive impairment responses are important research topics in the study of degenerative brain diseases as well as in understanding of human mental activities. To compare response to scopolamine (SPL)-induced cognitive impairment, we measured altered parameters for learning and memory ability, inflammatory response, oxidative stress, cholinergic dysfunction and neuronal cell damages, in Korl:ICR stock and two commercial breeder stocks (A:ICR and B:ICR) after relevant SPL exposure. In the water maze test, Korl:ICR showed no significant difference in SPL-induced learning and memory impairment compared to the two different ICRs, although escape latency was increased after SPL exposure. Although behavioral assessment using the manual avoidance test revealed reduced latency in all ICR mice after SPL treatment as compared to Vehicle, no differences were observed between the three ICR stocks. To determine cholinergic dysfunction induction by SPL exposure, activity of acetylcholinesterase (AChE) assessed in the three ICR stocks revealed no difference of acetylcholinesterase activity. Furthermore, low levels of superoxide dismutase (SOD) activity and high levels of inflammatory cytokines in SPL-treated group were maintained in all three ICR stocks, although some variations were observed between the SPLtreated groups. Neuronal cell damages induced by SPL showed similar response in all three ICR stocks, as assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, Nissl staining analysis and expression analyses of apoptosis-related proteins. Thus, the results of this study provide strong evidence that Korl:ICR is similar to the other two ICR. Stocks in response to learning and memory capacity.
Learning and memory are basic elements of cognitive behavior. Learning is the acquisition of information and technology, whereas memory is defined as the process of preserving the acquired information and technology, and is considered a higher activity from simple synapse neural connections to psychological processes [12].
Recently, advances in medical technology and rapid aging has raised an interest in geriatric diseases. Among age-related disorders, neural degenerative disorders such as dementia, increasingly result in memory loss, abnormal behavior, personality changes and ultimately death [3]. Considering learning and memory, neurodegenerative disorders are closely related to brain disorders, and are characterized by memory, judgment, direction, comprehension, learning abilities and disorders of various cortical functions, including language [4]. Moreover, death of abnormal cells due to aging and reduction of nerve cell activity are thought to be the main causes of neurodegeneration [56].
A variety of neuroscientific studies, ranging from geriatric diseases to understanding learning and memory and developing drugs for brain neurological disorders, require a model that can assess behaviors ranging from learning and memory to the molecular level of brain cells. Animal models using rodents having well-defined methods of evaluating behavior of individual animals as well as assessing the molecular level of brain cells, are therefore considered suitable animal models [7].
One of the most common pharmacological models used in studies of degenerative brain diseases and learning and memory studies in animal models (including rodents) is the scopolamine (SPL)-induced memory disorder model. This model has been applied for many years in understanding degenerative brain diseases, drug development and drug evaluation, and extensively studied as a tool for assessing various tasks such as passive avoidance, Y-Miro and Morris water maze [8]. It is well documented that selective muscarinic antagonists differentially affect the in vivo acetylcholine release and memory performance of young and aged rats. [9101112]. SPL is a frequently used muscarinic receptor antagonist in learning and memory impairment animal models. It blocks the connection between acetylcholine and muscarinic receptors, thereby temporarily blocking the information transmission, and subsequently resulting in learning and memory impairment [13].
Numerous studies report that in animal models used in neurobiological studies (particularly mouse studies) the alterations in neurobiological characteristics are observed (including behavior) due to genetic differences in mouse strains [14151617]. Inbred mice such as C57BL/6 and BALB/c are therefore consistently used to ensure genetically controlled and reliable animal experiments [18]. Conversely, out-bred mice such as ICR mice received less consideration as their variable genetic background results in difficulties for experimental measurements. Clinically, it is important to have a variety of genetic characteristics, and out-bred mouse models are required due to their inherent characteristics such as easy breeding and resistance to diseases [14].
This study compared the SPL-induced responses for cognitive impairment between the Korl:ICR stock and two commercial ICR stocks, to verify the characteristics of Korl:ICR mice as established by the Korea FDA. Our study results provide various scientific evidences of similar responses to SPL-induced cognitive impairment, as observed in the brain of Korl:ICR, A:ICR and B:ICR stocks, although slight differences were seen in the magnitude of these effects.
All animal protocols used in this study were reviewed and approved by the PNU-Institutional Animal Care and Use Committee (PNU-IACUC, approval number PNU-2017-1613). Male ICR mice (6-weeks-old) were obtained from three different sources. The Korl:ICR mice were kindly provided by the Department of Laboratory Animal Resources of the National Institute of Food and Drug Safety Evaluation (NIFDS, Chungju, Korea). The other two groups of ICR mice (A:ICR and B:ICR) were purchased from vendors located in the United States (Vendor A) and Japan (Vendor B), respectively. All ICR mice were maintained and treated at the Animal Resource Center of Pusan National University, which is certified by the Food and Drug Administration (FDA) as accredited unit number 000231, and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525). During the experiment, all mice were maintained in a specific pathogen-free (SPF) state under a strict light cycle (lights on at 08:00 h and off at 20:00 h), at 23±2℃ and 50±10% relative humidity. Animals were provided with ad libitum access to a standard irradiated chow diet (Samtako Inc.).
Cognitive impairment was induced as previously described [19]. The ICR mice (n=64) were first divided into two groups for Morris water maze test (n=32) and passive avoidance test (n=32). Briefly, 8-week-old ICR mice in each group (n=32) were assigned to either a non-impairment group (Vehicle-treated group, n=8) or an impairment group (n=24). The impairment group was further subdivided into Low SPL-treated (n=8), Mid SPL-treated (n=8), and Hi SPL-treated (n=8) groups. Appropriate amount (0.5, 1 and 2 mg/kg) of SPL (Sigma-Aldrich Co., Missouri, USA) was additionally intraperitoneally injected into each SPL groups for experimental conditions. Brain samples were collected of all mice applied to the behavioral test, after being subjected to euthanasia using a chamber filled with CO2 gas.
The Morris water maze experiment was conducted in a circular pool (diameter 180 cm, height 75 cm) with water maintained at 21±2℃ [20]. The pool was divided into quadrants and the hidden escape platform was placed 1 cm below the water surface in one of the quadrants. During the next 5 days, the mice swam three times a day (180 seconds each) over the platform. In the event of discovering the platform, the mice were allowed to remain on the platform for 20 seconds. The time required to find the hidden platform (latency) during each test period was recorded using a video tracking system. After the 5 day training session, the swimming time of the mouse was recorded on the 6th day with the escape platform removed from the pool. In the case of SPL-treated groups, SPL was administered with I.P. injection 30 minutes prior to start of training.
Passive avoidance test is a well-established experiment that evaluates short-term memory in studies of learning and memory [2122]. Two compartments are made in the same chamber (Gemini Avoidance System, San Diego, Calif., USA): one is a well-illuminated compartment and the other non-illuminated compartment is floored with a stainless-steel bar; both compartments are separated by a guillotine door. The target mouse is initially placed in the light compartment, and they enter the dark compartment in accordance with the inherent tendency of rodents. The separating door is locked and a mild electric shock (75 V, 0.5 mA, 50 Hz) is sustained for 5 seconds through the stainless-steel rod. Eight hours after the initial shock therapy, the target mouse was placed back in the illuminated compartment, and time taken for the mouse to enter the non-illuminated compartment was measured up to 300 seconds, and determined as the latency in the acquisition and retention tests.
The mRNA expression of IL-1β and COX-2 were measured using RT-PCR. Total RNA was extracted from the brain tissue of all experimental animals with RNAzol CS104 (Tel-Test Inc., Friendswood, USA). The separated mRNA was reverse transcribed using M-MLV reverse transcriptase (Promega, WI, USA) at 42℃ for 1 hour according to the manufacturer's protocol. Next, 10 pmol of the sense and antisense primers were added, and the reaction mixture was subjected to 28–32 cycles of amplification. Amplification was conducted in a Perkin-Elmer Thermal Cycler using the following cycles: 30 sec at 94℃, 30 sec at 62℃, and 45 sec at 72℃. The primer sequences for target gene expression identification were as follows: COX-2, sense primer: 5′-CAG GTC ATT GGT GGA GAG GTG TAT C-3′, anti-sense primer: 5′-CCA GGA GGA TGG AGT TGT TGT AGA G-3′; IL-1β, sense primer: 5′-GCA CAT CAA CAA GAG CTT CAG GCA G-3′, anti-sense primer: 5′-GCT GCT TGT GAG GTG CTG ATG TAC-3′; β-actin, sense primer: 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ anti-sense primer: 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. The experiment was repeated three times, and all samples were analyzed in triplicate. The final PCR products were separated on 1–2% agarose gel, followed by visualization after ethidium bromide staining. The density of a specific band was quantified using the Kodak Electrophoresis Documentation and Analysis System 120 (Eastman Kodak, Rochester, NY).
SOD activity in the brain tissue was estimated using the SOD Assay Kit (Dojindo Molecular Technologies Inc., Japan). Briefly, brain tissues of mice from each group were homogenized in 500 µL of sucrose buffer (0.25mol/L Sucrose, 10mmol/L HEPES, 1mmol/L EDTA, pH 7.4). The lysate was harvested after centrifugation (10,000 ×g, 60 min) and diluted with dilution buffer or saline as follows: 1, 1/5, 1/52, 1/53, 1/54, 1/55, and 1/56. For the enzyme reaction, 20 µL aliquot of each sample solution was placed in 96-well plates, to which 200 µL of the WST working solution was added. In addition, an enzyme working solution (20 µL) was added to each well and the samples were mixed thoroughly. The enzyme reaction was incubated at 37℃ for 20 min. Finally, absorbance was measured using a spectrophotometer at 450 nm. The SOD activity was calculated directly, using the following equation: SOD activity (inhibition rate %) =[(Ablank 1−Ablank 3)(Asample−Ablank 2)]/(Ablank 1−Ablank 3)×100 where, Ablank 1: absorbance of blank 1, Ablank 2: absorbance of blank 2, Ablank 3: absorbance of blank 3, Asample: absorbance of sample.
AChE activity in the brain tissue was determined using the Acetylcholinesterase Assay Kit (Abcam, Cambridge, UK). Brain tissue of mice from each group was homogenized in 0.1M phosphate buffer (Sigma-Aldrich Co.) and stored at −70℃ until analysis. The sample or standards and AChE reaction mixture were then incubated in a 96-well plate for 20 min at room temperature in the dark. Color alterations were read using a Versa max plate reader (Molecular Devices, Sunnyvale, CA, USA) at 405 nm.
DNA fragmentation was measured in the brain tissue using the TUNEL detection kit (BD Bioscience, Franklin Lakes, NJ, USA). After harvesting the brain tissue from each group, the tissues were embedded in paraffin; serial sections 4 µm thick were placed onto slides and stained with hematoxylin and eosin. For the TUNEL assay, sections of the brain were deparaffinized, and apoptotic cells were detected using the specific antibody for fragmented DNA. The percentage of FITC-BrdU positive cells and mean green fluorescence intensity were detected using Moticam pro 285A (Motic, Xiamen, China). All experiments were performed in duplicate.
Nissl staining was performed as previously described [23]. Briefly, brain tissues procured from the mice were placed in neutral buffered formaldehyde for fixation and perfused with 1× PBS. After perfusion, they were fixed overnight in neutral buffered formaldehyde, dehydrated and paraffin embedded. Brain sections (10 µm) of paraffin embedded tissues were cut using a Leica microtome (Leica Microsystems, Bannockburn, IL, USA). Deparaffinized with xylene for Nissl staining, rehydrated with ethanol at decreasing concentrations from 100–70%, and finally washed with distilled water (dH2O). Slides with brain sections were added to Nissl staining solution containing 0.1% cresyl violet acetate for 8 minutes and then washed with dH2O. The dead neurons were evaluated using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
Total proteins prepared from the brain tissue were separated by 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 2 h, after which resolved proteins were transferred to nitrocellulose membranes for 2 h at 40 V. Each membrane was then incubated separately, overnight at 4℃, with the following primary antibodies: anti-Bcl2 (Abcam), anti-Bax (Abcam), and anti-β-actin antibody (Sigma-Aldrich Co.). The membranes were then washed with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 0.05% Tween 20) and incubated with HRP-conjugated goat anti-rabbit IgG (Invitrogen, California, USA) and HRP-conjugated goat anti-mouse IgG (Invitrogen) at a 1:1,000 dilution, at room temperature for 1 h. Membrane blots were developed using Amersham ECL Select Western Blotting detection reagent (GE Heath care, Little Chalfont, UK).
Statistical analyses were performed with SPSS for Windows, release 10.10, standard version (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance followed by Tukey's post hoc test for multiple comparisons was performed to identify significant differences between groups. All values are reported as the mean±standard deviation (SD) and a P value <0.05 is considered as significant.
In order to compare the responses of SPL-induced spatial learning impairment among the three ICR stocks, the Morris water maze test was employed to determine the escape latency after exposure to three different concentrations of SPL. After the last day of the experimental period, the escape platform was removed and the free-swimming test was performed for the mouse under evaluation, to measure the first reaching time in the target quadrants. Figure 1 shows the time spent for the mouse to reach the quadrant where the escape platform was removed. The reaching time of the group treated with SPL was slower than the Vehicle group, and this tendency was similar in all three ICR stocks. We further compared the short- or long-term memory responses between the three ICR stocks after exposure to three different SPL doses, using the passive avoidance test. Compared to the Vehicle group, the time spent in the light compartment tended to decrease for all three ICR stocks after SPL treatment, and there were no significant differences between ICR stocks (Figure 2). These results show that the responses of Korl:ICR stock mice to SPL-induced learning and memory impairment were similar to the responses observed in the two commercial stocks.
To compare the differences in responses of the three ICR stocks to SPL-induced cholinergic dysfunction of the brain, the activity of AChE was assessed in brain tissues extracted from each ICR stock treated with three different doses of SPL. As presented in Figure 3, enhanced level of AChE activity was observed in the SPL-treated group as compared to the Vehicle group, with some differences observed in the increase rate between groups. These results suggest that responses of Korl:ICR stock to SPL-induced cholinergic dysfunction were similar to the two commercial stocks.
To compare the SPL-induced inflammation response and oxidative stress impairment of the Korl:ICR stock and the two commercial ICR stocks, we measured the levels of oxidative stress enzyme and inflammatory cytokines in the brain tissues extracted from the three ICR stocks treated with varying doses of SPL. We observed decreased activity of SOD in all SPL-treated groups as compared to Vehicle group. These patterns were commonly observed in all three stocks, with some differences between each group (Figure 4).
Next, RT-PCR analysis was performed to compare the SPL-induced inflammatory response in brain tissue of all ICR stocks since inflammation is known to result in learning and memory capacity impairment. The expressions of IL-1β (an inflammatory cytokine) and COX-2 (an inflammation-related protein) were found to be higher in all SPL-treated groups as compared to the respective Vehicle groups. However, the enhanced levels of expression were similar among the three ICR stocks (Figure 5). Overall, these results suggest that the inflammatory response and oxidative stress impairment exhibited by the Korl:ICR stock to SPL exposure were similar to responses of the two commercial stocks.
Numerous studies have reported that one of the major mechanisms of learning and memory impairment in SPL-treated animal models was the apoptotic induction of neuronal cells in the brain. We therefore examined the survival or modification of neuronal cells, DNA fragmentation and expression of apoptosis-related proteins in order to compare the responses of the Korl:ICR and two commercial stocks, to SPL-induced neuronal cell death using Nissl staining, TUNEL assay and western blot analysis. We observed significantly increased levels of fragmented DNA in all SPL-treated groups as compared to their Vehicle group. These patterns were similarly detected in all three ICR stocks (Figure 6).
Next, in order to compare the degree of SPL-induced neuronal cell death in the three ICR stocks, the number of dead neuronal cells were evaluated after Nissl staining of brain tissues obtained from each of the tested ICR mice. Nissl staining is a well-known method used to specifically stain the Nissl body, a large granular body found in neurons, and to identify neuronal cell death [24]. The area of the hippocampus shown in each panel of low resolution for structural observing was closely observation was further observed at higher resolution (Figure 7). Compared with the neural staining of brain tissues obtained from the Vehicle control, the brain tissues of each SPL-treated ICR stocks were abundant in dark blue stained cells in the hippocampal CA1 and CA3 regions. However, the number of these cells did not differ between the three ICR stocks (Figure 7). Similar results are observed in Figure 8, wherein we present the expression levels of Bax and Bcl-2 (both apoptosis-related proteins) after SPL exposure. These results indicate that the increase in apoptosis of brain cells, which is a mechanism of learning and memory animal models induced by SPL, do not differ much when comparing the three ICR stocks.
In our current study, SPL was used to induce animal models for dementia, such as Alzheimer's disease, to compare the response and mechanism of learning and memory in Korl:ICR and ICR procured from other sources.
SPL competitively inhibits the binding of acetylcholine to muscarinic receptors and is known to cause cholinergic depression [9101112]. Furthermore, SPL stimulates reactive oxygen species (ROS) to induce free radical damage, leading to oxidative stress [2526]. Many clinical studies have reported that oxidative stress is closely related to the pathogenesis of Alzheimer's disease. [27282930]. SPL administration significantly reduce the enzymatic activity of SOD and catalase, the two most important antioxidant enzymes [3132]. It has also been reported that SPL induces a high level of oxidative stress and promotes the inflammatory response by promoting the production of inflammatory cytokines [3334]. The development of inflammation in the nervous system results in the death of nerve cells, leading to deterioration of learning and memory capacity, including degeneration of nerve cells [3536]. The SPL administration model for animals is well known as a useful means of exploring the basic knowledge of learning and memory response, as well as developing new drugs towards neurodegenerative diseases such as Alzheimer's disease, which has inherent characteristics of problems with learning and memory response [9103437]. Clinical assessment of learning and memory ability is relatively easy to evaluate for humans. However, unlike methods of evaluating humans, more specific methods such as open field testing, passive avoidance and the Morris water maze are used in animals [3839]. The well-known and preferred assessment method in animal models of learning and memory abilities is the Morris water maze, rather than open-field testing and passive avoidance responses due to variation factors such as anxiety, depressive emotions and pain. Using learning and memory response, the Morris water maze assesses spatial learning and memory ability by measuring the swimming time of target animals in a pool, and the time spent in a particular area to find the supporting plate. SPL was reported to cause anomalies in acquisition, immediate maintenance, and working memory [404142], and it is well known that SPL impairs learning and memory processing by functioning as a cholinergic receptor antagonist [4344]. Several studies report that Morris water maze testing is capable of detecting cholinergic changes [4546]. Therefore, in the current study, learning and memory ability among the three ICR stocks was evaluated by applying the Morris water maze, as presented in Figure 1.
Kim et al. reported that compared to the in-bred mice, C57BL/6 and BALB/c mice, the out-bred ICR mice were more sensitive to evaluating the learning and memory responsiveness, but were slightly lower in memory ability [47]. ICR and BALB/c took longer to learn the same working memory than C57BL/6 mice in the Morris water maze. These differences are reported to be due to differences in visual abilities, with C57BL/6 mice showing normal visual acuity whereas ICR and BALB/c exhibit poor visual performance [1416]. Genetically well-controlled in-bred mice have traditionally been used in learning and memory studies. Recently, out-bred mice have also been favored since they represent a clinical manifestation of genetic diversity, similar to human.
In conclusion, we identified the conditions for establishing animal models for learning and memory by administering SPL in ICR mice. We examined the differences of cognitive-behavioral response in SPL-induced learning and memory of animal models among three ICR stocks, namely, Korl: ICR, A:ICR, and B:ICR. Moreover, we found that cholinergic reactions, oxidative stress, inflammation and apoptosis of neuronal tissue after SPL exposure were similar for all three ICR stocks. Our results suggest that ICR mice from other commercial suppliers, as well as the Korl:ICR mice, can be extensively applied to produce learning and memory animal models using SPL.
Figures and Tables
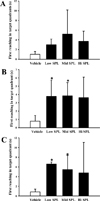 | Figure 1Comparison of the three ICR stocks on SPL-induced impairment of learning and memory in the Morris water maze. The first reaching time was evaluated in the target quadrant of the pool among Korl:ICR (A), A:ICR (B), and B:ICR (C) mice after exposure to SPL or Vehicle. Each first reaching time was evaluated as described in Materials and Methods. Data represents the mean±SEM of n=8/group (*P<0.05 versus Vehicle control group). |
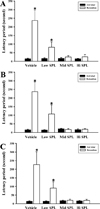 | Figure 2Comparison of behavioral responses of the three ICR stocks towards SPL-induced learning and memory impairment in the passive avoidance test. Panel represents the latency period into the dark chamber for Korl:ICR (A), A:ICR (B), and B:ICR (C) mice after treatment with SPL or Vehicle. Each latency period was evaluated as described in Materials and Methods. Data represents the mean±SEM of n=8/group (*P<0.05 versus Vehicle control group). |
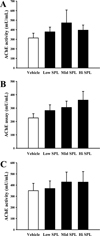 | Figure 3Comparison of AChE activity in SPL treated brain samples among the Korl:ICR, A:ICR, and B:ICR mice. Relative AChE activity levels were measured using the Acetylchol-inesterase Assay Kit, according to the experimental procedure described in the Materials and Methods. A, B, and C plots present the relative AChE activity of brain tissue from Korl:ICR, A:ICR, and B:ICR stocks, respectively. Data represents the mean±SEM of n=8/group (*P<0.05 versus Vehicle control group). |
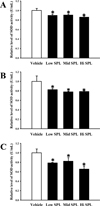 | Figure 4SOD activity was measured in the homogenate of the brain tissue collected from SPL treated ICR stocks. Each panel represents the SOD activity of Korl:ICR (A), A:ICR (B), and B:ICR (C) mice after treatment with SPL or Vehicle. Data represents the mean±SEM of n=8/group (*P<0.05 versus Vehicle control group). |
 | Figure 5Differing inflammatory responses of SPL-treated brain tissue among the three ICR stocks. The mRNA levels of inflammation related proteins (IL-1β and COX-2) were measured by RT-PCR using specific primers. Amplified DNA products were electrophoresed on the agarose gel and the intensity of the specific bands was recorded by a gel documentation system. Each panel represents the mRNA expression level of inflammation related proteins among Korl:ICR (A), A:ICR (B), and B:ICR (C) mice after treatment with SPL or Vehicle (*P<0.05 versus Vehicle control group). |
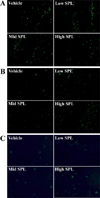 | Figure 6Determination of SPL-induced apoptosis among the three ICR stocks using TUNEL assay. Representative images show staining of TUNEL in brain tissues treated with Vehicle or SPL, as indicated. (A), (B), and (C) panel display fluorescence of FITC-BrdU on brain tissue from Korl:ICR, A:ICR, and B:ICR stocks, respectively. |
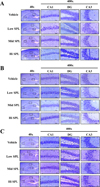 | Figure 7Comparison of SPL-induced apoptosis among Korl:ICR, A:ICR, and B:ICR mice using Nissl staining. This staining represent apoptotic cells in the hippocampus of the brain tissue from each ICR stock. Low intensity was observed in the hippocampus (4× magnification). Detailed histological features of several regions of the hippocampus are shown at 400× magnification. (A), (B), and (C) panels display the Nissl staining outcome on brain tissues from Korl:ICR, A:ICR, and B:ICR stocks, respectively. |
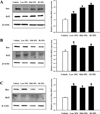 | Figure 8Determination of SPL-induced apoptosis among the three ICR stocks by comparing the Bax/Bcl-2 protein ratio. Total tissue lysates were prepared from the brain tissues of SPL-treated Korl:ICR (A), B:ICR (B), and B:ICR (C) mice of each group, as described in Materials and Methods. A total of 50 µg of protein per sample was immunoblotted with antibodies for each protein. Three samples were assayed in triplicate by western blotting. Data represents the means±SEM of three replicates (*P<0.05 versus Vehicle control group). |
Acknowledgments
We thank the animal technician, JinHyang Hwang, for directing the animal care and use at the Laboratory Animal Resources Center. This project was supported by a 2017 grant of BIOREIN (Laboratory Animal Bio Resources Initiative) received from the Ministry of Food and Drug Safety.
References
2. Okano H, Hirano T, Balaban E. Learning and memory. Proc Natl Acad Sci U S A. 2000; 97(23):12403–12404.

3. Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005; 13(4):324–328.

4. Anderson LA, McConnell SR. Cognitive health: an emerging public health issue. Alzheimers Dement. 2007; 3:2 Suppl. S70–S73.

5. Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010; 40(1-2):211–216.
6. Xu Z, Li H, Jin P. Epigenetics-based therapeutics for neurodegenerative disorders. Curr Transl Geriatr Exp Gerontol Rep. 2012; 1(4):229–236.

7. Kim DH, Ryu JH. Differential effects of scopolamine on memory processes in the object recognition test and the morris water maze test in Mice. Biomol Ther. 2008; 16(3):173–178.

8. Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997; 79(3):837–846.

9. Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010; 649(1-3):210–217.

10. Lee YK, Yuk DY, Kim TI, Kim YH, Kim KT, Kim KH, Lee BJ, Nam SY, Hong JT. Protective effect of the ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. J Nat Med. 2009; 63(3):274–282.
11. Pachauri SD, Tota S, Khandelwal K, Verma PR, Nath C, Hanif K, Shukla R, Saxena JK, Dwivedi AK. Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: a behavioral, biochemical and cerebral blood flow study. J Ethnopharmacol. 2012; 139(1):34–41.
12. Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012; 232(1):66–76.

13. Tanabe F, Miyasaka N, Kubota T, Aso T. Estrogen and progesterone improve scopolamine-induced impairment of spatial memory. J Med Dent Sci. 2004; 51(1):89–98.
14. Adams B, Fitch T, Chaney S, Gerlai R. Altered performance characteristics in cognitive tasks: comparison of the albino ICR and CD1 mouse strains. Behav Brain Res. 2002; 133(2):351–361.

15. Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002; 136(2):489–501.

16. Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007; 14(3):134–144.

17. Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000; 7(3):170–179.

19. Song SH, Choi SM, Kim JE, Sung JE, Lee HA, Choi YH, Bae CJ, Choi YW, Hwang DY. α-Isocubebenol alleviates scopolamine-induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci Lett. 2017; 638:121–128.

20. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11(1):47–60.

21. Kim MJ, Choi SJ, Lim ST, Kim HK, Kim YJ, Yoon HG, Shin DH. Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem. 2008; 72(2):577–581.

22. LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993; 702:149–157.

23. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124.

24. Ooigawa H, Nawashiro H, Fukui S, Otani N, Osumi A, Toyooka T, Shima K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006; 112(4):471–481.

25. Balaban H, Nazýroðlu M, Demirci K, Övey ÝS. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2017; 54(4):2852–2868.

26. Wong-Guerra M, Jiménez-Martin J, Pardo-Andreu GL, Fonseca-Fonseca LA, Souza DO, de Assis AM, Ramirez-Sanchez J, Del Valle RM, Nuñez-Figueredo Y. Mitochondrial involvement in memory impairment induced by scopolamine in rats. Neurol Res. 2017; 39(7):649–659.

28. Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998; 150(1):40–44.

29. Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002; 2(3):120–123.

30. Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000; 1502(1):139–144.

31. Balu M, Sangeetha P, Haripriya D, Panneerselvam C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci Lett. 2005; 383(3):295–300.

32. Mann H, McCoy MT, Subramaniam J, Van Remmen H, Cadet JL. Overexpression of superoxide dismutase and catalase in immortalized neural cells: toxic effects of hydrogen peroxide. Brain Res. 1997; 770(1-2):163–168.

33. Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002; 133(2):369–376.

34. Lee B, Shim I, Lee H, Hahm DH. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. 2011; 21(8):874–883.

35. Choi DY, Lee YJ, Lee SY, Lee YM, Lee HH, Choi IS, Oh KW, Han SB, Nam SY, Hong JT. Attenuation of scopolamine-induced cognitive dysfunction by obovatol. Arch Pharm Res. 2012; 35(7):1279–1286.

36. Liskowsky W, Schliebs R. Muscarinic acetylcholine receptor inhibition in transgenic Alzheimer-like Tg2576 mice by scopolamine favours the amyloidogenic route of processing of amyloid precursor protein. Int J Dev Neurosci. 2006; 24(2-3):149–156.

37. Gattu M, Boss KL, Terry AV Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997; 57(4):793–799.
38. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124.

39. Yoneoka Y, Satoh M, Akiyama K, Sano K, Fujii Y, Tanaka R. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999; 72(864):1196–1201.

40. Beninger RJ, Jhamandas K, Boegman RJ, el-Defrawy SR. Effects of scopolamine and unilateral lesions of the basal forebrain on T-maze spatial discrimination and alternation in rats. Pharmacol Biochem Behav. 1986; 24(5):1353–1360.

41. Carballo-Márquez A, Vale-Martínez A, Guillazo-Blanch G, Torras-Garcia M, Boix-Trelis N, Martí-Nicolovius M. Differential effects of muscarinic receptor blockade in prelimbic cortex on acquisition and memory formation of an odor-reward task. Learn Mem. 2007; 14(9):616–624.
42. Halder S, Mehta AK, Kar R, Mustafa M, Mediratta PK, Sharma KK. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011; 77(8):830–834.

43. Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004; 20(4):1081–1088.

44. Liem-Moolenaar M, de Boer P, Timmers M, Schoemaker RC, van Hasselt JG, Schmidt S, van Gerven JM. Pharmacokinetic-pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol. 2011; 71(6):886–898.

45. Hosseini-Sharifabad A, Mohammadi-Eraghi S, Tabrizian K, Soodi M, Khorshidahmad T, Naghdi N, Abdollahi M, Beyer C, Roghani A, Sharifzadeh M. Effects of training in the Morris water maze on the spatial learning acquisition and VAChT expression in male rats. Daru. 2011; 19(2):166–172.
46. Lamberty Y, Gower AJ. Cholinergic modulation of spatial learning in mice in a Morris-type water maze. Arch Int Pharmacodyn Ther. 1991; 309:5–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download