Abstract
Nucleotide-binding domain 1 (Nod1) is a cytosolic receptor that is responsible for the recognition of a bacterial peptidoglycan motif containing meso-diaminophimelic acid. In this study, we sought to identify the role of Nod1 in host defense in vivo against pulmonary infection by multidrug resistant Acinetobacter baumannii. Wildtype (WT) and Nod1-deficient mice were intranasally infected with 3×107 CFU of A. baumannii and sacrificed at 1 and 3 days post-infection (dpi). Bacterial CFUs, cytokines production, histopathology, and mouse β-defensins (mBD) in the lungs of infected mice were evaluated. The production of cytokines in response to A. baumannii was also measured in WT and Nod1-deficient macrophages. The bacterial clearance in the lungs was not affected by Nod1 deficiency. Levels of IL-6, TNF-α, and IL-1β in the lung homogenates were comparable at days 1 and 3 between WT and Nod1-deficient mice, except the TNF-α level at day 3, which was higher in Nod1-deficient mice. There was no significant difference in lung pathology and expression of mBDs (mBD1, 2, 3, and 4) between WT and Nod1-deficient mice infected with A. baumannii. The production of IL-6, TNF-α, and NO by macrophages in response to A. baumannii was also comparable in WT and Nod1-deficient mice. Our results indicated that Nod1 does not play an important role in host immune responses against A. baumannii infection.
Acinetobacter baumannii is a rod-shaped, non-motile gram-negative bacterium. Whereas other Acinetobacter spp. are naturally isolated in soil, water and animal habitats, A. baumannii is intensely isolated in the hospital environment [12]. It causes opportunistic infections in patients with underlying diseases and immunosuppression, which leads to various diseases, such as pneumonia, bacteremia, endocarditis, skin and soft-tissue infections, urinary-tract infection, and meningitis [3]. This bacterium is also referred to as the ‘Iraq bacter’ because it has exploded in US military hospital patients during the Iraq war [4].
About 30 years ago, A. baumannii infections were treated with traditional antibiotics [5]. However, it is now resistant to almost all major antibiotics, including penicillins, carbapenems, and cephalosporins [5]. The increase of these multidrug-resistant (MDR) bacteria is becoming a serious clinical problem, and the incidence is rapidly increasing worldwide. A. baumannii is listed on the World Health Organization's catalog of bacteria for which new treatments are critically needed [6]. Despite its clinical importance, there has been little progress in developing a control strategy for this new threat.
Nucleotide-binding oligomerization domain (Nod)-like receptors (NLRs) are a family of cytosolic receptors that can recognize microbial or damage-associated molecules [7]. Among them, Nod1 and Nod2 are the first identified NLRs and recognize bacterial peptidoglycan derivatives, meso-diaminophimelic acid (DAP) and muramyl dipeptide (MDP), respectively [8]. It has been known that Nod1 is ubiquitously expressed, whereas Nod2 is mostly in immune cells [9]. After recognition, they recruit and associate with an adaptor protein receptor interacting serine/threonine-protein kinase 2 (Ripk2), which activates downstream signaling, such as nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) [7910]. These events are finally involved in host defense against microbial infections by producing cytokines or inflammatory mediators, such as reactive oxygen species (ROS) [1112].
It has been widely known that Nod1 and Nod2 regulate innate immune response and protect hosts against pulmonary bacterial infections [11131415]. However, little is known about the role of the Nod1 and Nod2 pathways in host immune response against A. baumannii infection. A previous study revealed that the Nod1/2-Ripk2 pathways control intracellular growth of A. baumannii in a human lung epithelial-cell line, A549 cells [16]. The signaling is also essential for NF-κB activation and β-defensin expression in the cells in response to A. baumannii [16]. An in vivo study showed that Nod2 contributes to bacterial clearance and ROS production in the lungs of mice infected with A. baumannii at an early phase of infection [17]. However, we still do not know the function of Nod1 in control of A. baumannii infection in vivo. In this study, we investigated the in vivo role of Nod1 in control of bacterial growth and immune responses in the lungs of mice infected with A. baumannii.
WT C57BL/6 mice were obtained from Koatech (Pyeongtaek, Korea). Nod1−/− mice were gifts from Prof. Gabriel Núñez (University of Michigan, USA) and have been previously described [18]. Protocols for animal studies were approved by the Institutional Animals Care and Use Committee of Chonnam National University (Approval No: CNU IACUC-YB-R-2017-76).
A. baumannii strain KCCM 35453 (ATCC 15150) was purchased from the Korean Culture Center of Microorganisms (Seoul, Korea). Single colonies were inoculated into 10 mL of Luria-Bertani (LB) broth supplemented with ampicillin (50 mg/mL) and grown overnight at 37℃ with 200 rpm shaking. A 1:5 dilution of the culture suspension was allowed to grow in fresh medium at 37℃ with 200 rpm shaking for an additional 2 h. Bacteria were washed and resuspended with sterile phosphate buffered saline (PBS) to a final concentration of 109 colony-forming units (CFU)/mL. Bacteria were diluted to desired concentrations for use in experiments.
Mice were anesthetized by intraperitoneal injection of 10 mg/kg Rompun (Bayer, Seoul, Korea) and 50 mg/kg Zoletil (Virbac, Seoul, Korea). They were then intranasally (i.n) inoculated with 30 µL of A. baumannii (1×109 CFU/mL) suspension in PBS. Lung homogenate was collected at 1 and 3 days post infection to quantify bacterial loads, cytokines production, and mouse β-defensins (mBD) expression.
Lung homogenates were spread onto LB agar plates supplemented with ampicillin (50 mg/mL). Following overnight culture at 37℃ in an incubator, bacterial colonies were counted, and the number of bacteria was expressed as CFU/g of lung tissue.
Concentrations of IL-6, TNF-α, and IL-1β from the lung homogenates of A. baumannii-infected mice or culture supernatant of macrophages were measured using ELISA kits (R&D System, Minneapolis, MN, USA) according to the manufacturer's instructions.
The left lobe of the lung was harvested and fixed in 10% neutral formalin for histopathological observation. The tissues were routinely processed with alcohol and xylene series and embedded in paraffin. Three-micrometer sections were prepared, stained with hematoxylin-eosin (HE), and examined by microscopy. Histopathology of the lung was blindly evaluated using an arbitrary scoring system according to described in a previous study [19].
Total RNA was extracted from lung tissue using easy-BLUE (Intron Biotechnology, Korea) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed to cDNA by using ReverTra Ace qPCR RT Master Mix and cDNA Synthesis kit (Toyobo, Osaka, Japan). Equal amounts (1 µL) of cDNA were used for real-time PCR on a Rotor-Gene Q (Qiagen, Hilden, Germany) using SYBR Green PCR kit (Qiagen). GAPDH was used for normalization. The following primers were used for real time PCR: GAPDH forward: 5′-CAGTGGATGCAGGGATGATGTTCT-3′; GAPDH reverse: 5′-GTGGAGATTGTTGCCATCAACG-3′; mBD-1 forward: 5′-CCAGATGGAGCCAGGTGTTG-3′; mBD-1 reverse: 5′-AGCTGGAGCGGAGACAGAATCC-3′; mBD-2 forward: 5′-AAGTATTGGATACGAAGCAG-3′; mBD-2 reverse: 5′-TGGCAGAAGGAGGACAAATG; mBD-3 forward: 5′-GCATTGGCAACACTCGTCAGA-3′; mBD-3 reverse: 5′-CGGGATCTTGGTCTTCTCTA-3′; mBD-4 forward: 5′-GCAGCCTTACCCAAATTATC-3′; mBD-4 reverse: 5′-ACAATTGCCAATCTGTCGAA-3′.
Bone marrow-derived macrophages (BMDMs) were prepared as previously described [20]. The cells were seeded in 48-well plates at the concentration of 2×105/well and incubated in a 5% CO2 incubator at 37℃ overnight. Subsequently, cells were either infected or not infected with A. baumannii at the indicated multiplicity of infection (MOI) by exposure for 60 min, and extracellular bacterial growth was inhibited by gentamicin (50 µg/mL) treatment. Culture supernatant was collected 24 h after infection for cytokine measurement.
Difference between groups was assessed by two-tailed Student's t-test or one-way analysis of variance followed by post hoc analysis (Newman-Keuls multiple comparison test). All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was considered at P<0.05.
WT and Nod1-deficient mice were intranasally infected with A. baumannii and the bacterial CFUs were measured in the lungs at 1 and 3 days postinfection (dpi). The experiment was repeated twice independently. The bacterial CFUs in the lungs were over 8 log10CFU/g lung at 1 dpi and decreased by 4 log10CFU/g lung at 3 dpi in both WT and Nod1-deficient mice (Figures 1A, B). There was no significant difference of the bacterial CFUs in the lungs between WT and Nod1-deficient mice (Figures 1A, B).
The level of cytokines in the lung homogenates was measured by ELISA. IL-6 and TNF-α levels in WT and Nod1-deficient mice infected with A. baumannii were reduced more on 3 dpi than on day 1, whereas IL-1β production was increased at 3 dpi (Figures 2A–C). Except for TNF-α at day 3, the level of which was higher in the lung homogenate of Nod1-deficient mice, the levesl of each cytokine were comparable between WT and Nod1-deficient mice at 1 or 3 dpi (Figures 2A–C).
We next examined the role of Nod1 in A. baumannii-induced lung pathology in mice. Intranasal infection of A. baumannii led to moderate to severe lung pathology, which was characterized by neutrophilic infiltration and edematous lesion (Figure 3A). The lung pathology was getting severe at day 3 compared to day 1 in both WT and Nod1-deficient mice (Figures 3A, B). When quantitatively evaluated, there was no significant difference of histological score in lung pathology between WT and Nod1-deficient mice (Figure 3B).
A. baumannii induce gene expression of human β-defensin 2 (hBD-2) in human airway epithelial cells, which is regulated by Nod1 and Nod2 signaling [1621]. Accordingly, we tried to find out whether Nod1 deficiency leads to impaired expression of β-defensins in vivo. mBD-1 expression in the lungs was reduced at days 1 and 3 after A. baumannii infection, compared to that in uninfected control animals (Figure 4A). In contrast, the gene expression of mBD-2 and mBD-3 was increased at day 1 and restored at day 3 (Figures 4B, C). There was no significant difference of gene expression of mBD-1, mBD-2, and mBD-3 in the lungs of WT and Nod1-deficient mice (Figures 4A–C). mBD-4 expression, a mouse orthologue of hBD-2, was also decreased in WT mice at days 1 and 3 compared to the uninfected controls, whereas it did not change in Nod1-deficient mice (Figure 4D).
Macrophages play a role in early host resistance to pulmonary infection by A. baumannii [22], and Nod1 is also functional in macrophages [23]. We then investigated the role of Nod1 in the production of cytokines in response to A. baumannii in macrophages. A. baumannii induced the production of IL-6 and TNF-α in both WT and Nod1-deficient BMDMs without any difference in their levels between the two groups (Figures 5A–C).
The role of Nod2 in host defenses against respiratory bacterial infections has been widely studied, whereas studies about Nod1 are limited. Nod2 is knownt to contribute to host resistance against various respiratory pathogens, such as Staphylococcus aureus, Mycobacterium tuberculosis, M. abscessus, Streptococcus pneumoniae, and A. baumannii [131724252627]. Nod2 also has antiviral activity by regulating type I interferons production in response to ssRNA virus via a mitochondrial antiviral-signaling protein (MAVS)-dependent pathway [28]. In Legionella pneumophila-infected mice, Nod1 deficiency led to impaired neutrophilic recruitment to the lungs [1529]. In the study, a single deficiency of Nod1 or Nod2 did not influence the clearing of L. pneumophila from the lungs of mice at most time points [1529], except on day 3, at which the bacterial CFUs in the lungs were higher in Nod1-deficient mice than in WT mice [15]. However, the bacterial clearing was impaired in mice deficient in Ripk2 [29], which is an essential adaptor protein of both Nod1 and Nod2, indicating that Nod1 and Nod2 cooperate to control the growth of L. pneumophila in the lungs.
In our study, we have shown that Nod1 is not required to clear bacteria from the lungs of mice infected with A. baumannii. Nod1 deficiency did not affect in vivo cytokines production, lung pathology, or β-defensins expression in the lungs of infected mice. Moreover, A. baumannii-induced production of IL-6 and TNF-α was not impaired in Nod1-deficient macrophages. These results are similar to those in a study of Pseudomonas aeruginosa infection [30]. When infected with P. aeruginosa intranasally, neutrophils recruitment and production of chemokines, such as CXCL1 and CXCL2, in broncholavage (BAL) fluids were comparable between WT and Nod1-deficient mice [30]. Moreover, Nod1 is not involved in bacterial clearing in BAL fluids of mice infected with P. aeruginosa [30]. Taken toghether, Nod1 seems to not have a significant effect on host defense against acute respiratory infection of gram-negative bacteria with strong TLR4 activity.
There are some considerations about the possibility that Nod1 might not play an important role in host defense against pulmonary infection of A. baumannii. Although epithelial Nod1 is involved in β-defensin expression in response to the bacteria in vitro condition [16], our results showed that Nod1 is not needed for mBD-2 and mBD-3 expression in the lungs of infected mice. In addition, hBD-2 in human breast milk inhibits some bacterial growth, including Serratia marcescen and P. aeruginosa, with minimum inhibitory concentrations (MICs) of <0.5 mg/mL, whereas it showed a higher MIC (4 mg/mL) against a multidrug-resistant strain of A. baumannii [31], indicating that β-defensins may not be able to control in vivo growth of A. baumannii. Therefore, the effect of Nod1 on in vivo expression of β-defensins against A. baumannii is likely very limited, and at most, modest in the bacterial growth control by β-defensins.
We should also consider the role of Nod1 in the function of macrophages and neutrophils. At 4 h after A. baumannii infection, macrophages are dominant in BAL fluids, whereas neutrophils are at 24 h after infection [22]. A Nod1 ligand iE-DAP can induce the production of IL-6 and TNF-α in murine macrophages, but very low levels (<60 pg/mL) compared to those by LPS [23]. For bacteria possessing strong TLRs-stimulatory activity, it is likely that TLR2 or TLR4 masks the role of Nod1 and Nod2 in immune responses in macrophages. In fact, Nod1, Nod2, and Ripk2 are more critical for cytokines production in macrophages in response to several gram-negative bacteria when there is TLRs tolerization or deficiency [323334]. In this study, Nod1 deficiency does not influence cytokines production in A. baumannii-infected macrophages. Our previous study showed that A. baumannii-induced production of IL-6 and TNF-α was mostly abolished in TLR4-deficient macrophages [35]. Therefore, Nod1 is likely irrelevant in the immune response of macrophages against A. baumannii in the presence of TLR4.
There is a controversy about the effect of Nod1 in regulating neutrophil function. Clarke et al. showed that recognition of microbiota peptidoglycan by Nod1 strengthens neutrophil ability to kill some bacteria, such as S. pneumoniae and S. aureus [36]. However, Nod1 is not expressed in either human or mouse neutrophils, whereas a strong expression of Nod2 is observed [3738]. Nod2 agonist MDP induces cytokines and chemokines in neutrophils, but Nod1 stimulation does not [3738]. Further studies seems to be necessary to clarify the role of Nod1 in host defense by neutrophils against bacterial infection.
In conclusion, our study revealed that a single deficiency of Nod1 does not affect in vivo host defense against pulmonary infection of A. baumannii, although it is involved in NF-κB activation, hBD-2 expression, and restriction of the intracellular bacterial growth in respiratory epithelial cells against A. baumannii infection [16]. It cannot be excluded that Nod1 cooperates with Nod2 or TLRs to regulate host immune response against A. baumannii infection.
Figures and Tables
 | Figure 1Nod1 does not affect bacterial clearance in the lungs of A. baumannii-infected mice. WT and Nod1-deficient mice (n=5–6 mice per group) were intranasally (i.n) infected with A. baumannii (3×107 CFU) and sacrificed at 1 and 3 days after infection. Bacterial loads in lung homogenates were counted by agar plating assay. This experiment was repeated twice independently, and the results are provided separately (A and B). |
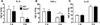 | Figure 2Cytokines production in the lung homogenates of WT and Nod1-deficient mice infected with A. baumannii. WT and Nod1-deficient mice (n=5–6 mice per group) were i.n infected with A. baumannii (3×107 CFU). Lung homogenates were collected at 1 and 3 days after infection. Levels of IL-6, TNF-α, and IL-1β were measured by ELISA (A–C). Results are from one of two independent experiments. |
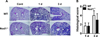 | Figure 3Lung pathology in WT and Nod1-deficient mice infected with A. baumannii. Lung pathology in WT and Nod1-deficient mice (n=5–6 mice per group) infected with A. baumannii was evaluated in H&E-stained section (A, ×40 magnification). Histology score was calculated as described in Materials and Methods, and the results are shown as mean±SD (B). |
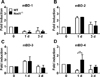 | Figure 4Expression of β-defensins in the lungs of WT and Nod1-dificient mice infected with A. baumannii. RNA was obtained from the lungs of WT and Nod1-deficient mice (n=5–6 mice per group) infected with A. baumannii. The gene expressions of mBD-1, -2, -3, and -4 were examined by real-time PCR (A–D). Fold increase (arbitrary unit) was obtained by comparing each level in the lungs from infected to that in uninfected control lungs. *P<0.05 and **P<0.01. |
 | Figure 5Nod1 deficiency does not influence the production of cytokines by macrophages in response to A. baumannii. WT and Nod1-deficient bone marrow-derived macrophages (BMDMs) in triplicates were infected with indicated doses of A. baumannii for 24 h, and levels of IL-6 and TNF-α in culture supernatants were measured by ELISA (A and B). Results are from one of three independent experiments. |
Acknowledgments
This study was supported by the Basic Research in Science and Engineering program, funded by the National Research Foundation of Korea (NRF) in the Ministry of Science and ICT of Korea (MSIT) (grant no. NRF-2018R1A2B3004143).
References
1. Doughari HJ, Ndakidemi PA, Human IS, Benade S. The ecology, biology and pathogenesis of Acinetobacter spp.: an overview. Microbes Environ. 2011; 26(2):101–112.

2. Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014; 71(3):292–301.

3. McConnell MJ, Actis L, Pachón J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013; 37(2):130–155.

4. Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007; 44(12):1577–1584.

5. Moradi J, Hashemi FB, Bahador A. Antibiotic Resistance of Acinetobacter baumannii in Iran: A Systemic Review of the Published Literature. Osong Public Health Res Perspect. 2015; 6(2):79–86.

6. Kale SD, Dikshit N, Kumar P, Balamuralidhar V, Khameneh HJ, Bin Abdul, Koh TH, Tan GGY, Tan TT, Mortellaro A, Sukumaran B. Nod2 is required for the early innate immune clearance of Acinetobacter baumannii from the lungs. Sci Rep. 2017; 7(1):17429.

7. Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009; 227(1):106–128.

8. Mathews RJ, Sprakes MB, McDermott MF. NOD-like receptors and inflammation. Arthritis Res Ther. 2008; 10(6):228.

9. Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014; 41(6):898–908.

10. Moreira LO, Zamboni DS. NOD1 and NOD2 Signaling in Infection and Inflammation. Front Immunol. 2012; 3:328.

11. Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, Arditi M. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 2009; 5(4):e1000379.

12. Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009; 122(pt19):3522–3530.

13. Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M, Cavaillon JM, Adib-Conquy M. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect. 2010; 12(10):759–767.

14. Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009; 5(7):e1000500.

15. Berrington WR, Iyer R, Wells RD, Smith KD, Skerrett SJ, Hawn TR. NOD1 and NOD2 regulation of pulmonary innate immunity to Legionella pneumophila. Eur J Immunol. 2010; 40(12):3519–3527.

16. Bist P, Dikshit N, Koh TH, Mortellaro A, Tan TT, Sukumaran B. The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection. Infect Immun. 2014; 82(3):1112–1122.

17. Kale SD, Dikshit N, Kumar P, Balamuralidhar V, Khameneh HJ, Bin Abdul Malik N, Koh TH, Tan GGY, Tan TT, Mortellaro A, Sukumaran B. Nod2 is required for the early innate immune clearance of Acinetobacter baumannii from the lungs. Sci Rep. 2017; 7(1):17429.

18. Kim YG, Park JH, Daignault S, Fukase K, Núñez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J Immunol. 2008; 181(6):4340–4346.

19. Kang MJ, Jo SG, Kim DJ, Park JH. NLRP3 inflammasome mediates interleukin-1β production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology. 2017; 150(4):495–505.
20. Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984; 160(1):55–74.

21. March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One. 2010; 5(4):e10033.
22. Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One. 2012; 7(6):e40019.

23. Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nuñez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003; 4(7):702–707.

24. Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. The J Clin Invest. 2011; 121(9):3666–9676.

25. Hommes TJ, van Lieshout MH, van 't Veer C, Florquin S, Bootsma HJ, Hermans PW, de Vos AF, van der Poll T. Role of Nucleotide-Binding Oligomerization Domain-Containing (NOD) 2 in Host Defense during Pneumococcal Pneumonia. PLoS One. 2015; 10(12):e0145138.

26. Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008; 181(10):7157–7165.

27. Lee JY, Lee MS, Kim DJ, Yang SJ, Lee SJ, Noh EJ, Shin SJ, Park JH. Nucleotide-Binding Oligomerization Domain 2 Contributes to Limiting Growth of Mycobacterium abscessus in the Lung of Mice by Regulating Cytokines and Nitric Oxide Production. Front Immunol. 2017; 8:1477.

28. Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009; 10(10):1073–1080.

29. Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sônego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. The pattern recognition receptors Nod1 and Nod2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect. 2010; 12(11):819–827.

30. Sônego F, Castanheira FVS, Horta CV, Kanashiro A, Czaikoski PG, Zamboni DS, Alves-Filho JC, Cunha FQ. The host control of a clinical isolate strain of P. aeruginosa infection is independent of Nod-1 but depends on MyD88. Inflamm Res. 2018; 67(5):435–443.

31. Baricelli J, Rocafull MA, Vázquez D, Bastidas B, Báez-Ramirez E, Thomas LE. β-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J Pediatr (Rio J). 2015; 91(1):36–43.

32. Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008; 28(2):246–257.

33. Park JH, Kim YG, Núñez G. RICK promotes inflammation and lethality after gram-negative bacterial infection in mice stimulated with lipopolysaccharide. Infect Immun. 2009; 77(4):1569–1578.

34. Jeong YJ, Kim CH, Kim JC, Oh SM, Lee KB, Park JH, Kim DJ. RIP2/RICK-dependent cytokine production upon Yersinia enterocolitica infection in macrophages with TLR4 deficiency. Scand J Immunol. 2013; 78(5):401–407.
35. Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR, Kang MJ, Park JH, Park JH. Essential role of toll-like receptor 4 in Acinetobacter baumannii-induced immune responses in immune cells. Microb Pathog. 2013; 54:20–25.

36. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010; 16(2):228–231.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download