Abstract
A few clues about correlation between endoplasmic reticulum (ER) stress and mulberry (Morus alba) leaves were investigated in only the experimental autoimmune myocarditis and streptozotocin-induced diabetes. To investigate whether a novel extract of mulberry leaves fermented with Cordyceps militaris (EMfC) could suppress ER in fatty liver, alterations in the key parameters for ER stress response were measured in high fat diet (HFD)-induced obese C57L/6 mice treated with EMfC for 12 weeks. The area of adipocytes in the liver section were significantly decreased in the HFD+EMfC treated group as compared to the HFD+Vehicle treated group, while their level was higher in HFD+Vehicle treated group than No treated group. The level of the eukaryotic initiation factor 2 alpha (eIF2α) and inositol-requiring enzyme 1 beta (IRE1α) phosphorylation and CCAAT-enhancer-binding protein homologous protein (CHOP) expression were remarkably enhanced in the HFD+Vehicle treated group. However, their levels were restored in the HFD+EMfC treated group, although some differences were detected in the decrease rate. Similar recovery was observed on the ER stress-induced apoptosis. The level of Caspase-3, Bcl-2 and Bax were decreased in the HFD+EMfC and HFD+orlistat (OT) treated group compared to the HFD+Vehicle treated group. The results of the present study therefore provide first evidence that EMfC with the anti-obesity effects can be suppressed ER stress and ER stress-induced apoptosis in the hepatic steatosis of HFD-induced obesity model.
ER stress is defined as an over accumulated condition of unfolded or misfolded proteins in ER lumen, leading to the activation of the unfolded protein response (UPR) [1]. As results of this response, abnormal molecular mechanisms such as attenuation of protein synthesis, induction of ER chaperone genes, increased ER-associated degradation (ERAD), and induction of apoptosis to safely dispose cells injured by ER stress can be induced in mammalian cells [12]. Also, the significant changes on the ER stress response was observed in several chronic diseases such as diabetes, cancer, atherosclerosis, inflammation and constipation as well as neurodegenerative diseases including Alzheimer's disease and Parkinson's disease [3456].
In adipose tissue, ER stress can be induced by various metabolism condition including nutrient overload, increasing demand for protein synthesis, regional glucose deprivation derived from insulin resistance, and decreasing vascularization [7]. This response was improved by some compounds and natural products with anti-obesity activity although there are differences in their efficacy. The treatment of magnesium lithospermate B, caffeic acid and taurine-conjugated derivative induced a significant decrease of ER stress marker level in HFD-induced obesity model [8910]. Also, nicotine improved ER stress and hepatic steatosis in the liver tissue of diet-induced obese model [11]. Meanwhile, mulberry leaf powder was reported as one of potential candidates with anti-ER stress activity. Diet containing 5% mulberry leaf powder attenuated the level of GRP78 and their downstream markers in experimental autoimmune myocarditis (EAM) and post myocarditis dilated cardiomyopathy (DCM) model [1213]. However, no studies have provided scientific evidence for the relief effects of fermented mulberry leaf on the various markers for ER stress, even though the effect of their unfermented products had been focused into the expression of GRP78 [1213].
In the present study, we investigated whether EMfC with anti-obesity activity could be relived the ER stress response in HFD-induced obesity model. These results provide the first evidence that EMfC may improve the ER stress and ER stress-induced apoptosis during the ingestion of HFD.
EMfCs used in this study were prepared in accordance with the previous papers [14]. Briefly, the samples of mulberry dry leaves were collected from plantations in the Sangju district of Korea in October 2015. These samples were confirmed to be mulberry leaves by Professor Young Whan Choi, one of the authors. Voucher specimens (accession number Mul-PDRL-1) were deposited in the herbarium of the Department of Horticultural Bioscience, the Pusan National University (Miryang, Korea). Also, the C. militaris used for fermentation was kindly provided Professor Sang Mong Lee of the Department of Life Science and Environmental Biochemistry, Pusan National University. The Jeongeup Agriculture Cooperative Federations for Silkworm Farming (Jeongeup, Korea) supplied the silkworm pupae powder.
To prepare the extracts of fermented mulberry powder, the powder of dry leaves was mixed with 50% silkworm powder (SWP), and the mixture inoculated with 10% C. military (v/w). These mixtures were incubated in a shaking incubator (#SI-600R, Lab Companion, Seoul, Korea) at 150 rpm and 25℃, and fermented for 4 weeks. After the harvest of fermented mixture, the extracted powder was mixed with the solvent (95% EtOH) in a fixed liquid ratio (mulberry powder:solvent, ratio 1:10) and sonicated for 1 hr using a JAC ultrasonic device (KODO, Hwangseong, Korea). The supernatant was separated from the sonicated extract using the centrifuging at 3,000 rpm for 10 min. The pellet was resuspended in 9 mL of the solvent, and further sonicated using the same conditions. This procedure was repeated once more, and the resultant supernatant was collected, filtered through a 0.4 µm filter (#HAWP04700, Millex-LH, MerckMillipore, Darmstadt, Germany), and evaporated using a vacuum evaporator (#R-300, BUCHI Corporation, New Castle, Delaware, USA). Finally, lyophilization of the EMfC was achieved using the circulating extraction equipment (IKA Labortechnik, Staufen, Germany). The extracts were dissolved in DMSO (#D2660, Sigma-Aldrich Co., St Louis, MO, USA) at a concentration of 50 mg/mL before use.
The protocol for animal study was approved by the Pusan National University Animal Ethics Committee (PNU-2017-1519). Eight-week-old C57BL/6 male mice were purchased from Samtako BioKorea Inc. (Osan, Korea), and provided ad libitum access to water and a standard irradiated chow diet (Samtako BioKorea Inc.). During the experiment, mice were maintained in a pathogen-free state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23±12℃ and 50±10% relative humidity. All C57BL/6 mice were handled at the Pusan National University-Laboratory Animal Resources Center, accredited by the Korea Food and Drug Administration (FDA) (Accredited Unit Number-000231) and AAALAC International (Accredited Unit Number; 001525).
All animals were acclimatized on a normal diet (D12450K; Research Diets, New Brunswick, NJ, USA) for 1 week. The C57BL/6 mice were then divided into 4 study groups (7 mice/group): (1) control group fed a normal diet (NO treated group), (2) group fed HFD (high fat diet) plus vehicle (olive oil+1% DMSO) (HFD+Vehicle treated group), (3) group fed HFD plus 10 mg/kg OT (Sigma-Aldrich Co.) (HFD+OT treated group), and (4) group fed HFD plus 50 mg/kg EMfC (HFD+EMfC treated group). For 12 weeks, mice of all HFD treatment groups consumed HFD containing 60% kcal fat purchased from Research Diets (#D12492, Research Diets, Inc., New Brunswick, USA). After 24 h of the final EMfC treatment, all mice were euthanized using CO2 gas, after which the tissue samples were acquired and stored in Eppendorf tubes at −70℃ until assay.
Liver tissues dissected from mice of all subset groups were fixed in 10% neutral buffered formaldehyde (pH 6.8) for 48 hr. The dehydrated liver tissue was then embedded in paraffin wax. Next, a series of liver and fat sections (4 µm) were cut from the paraffin-embedded tissues using a Leica microtome (#DM500, Leica Microsystems, Bannockburn, IL, USA). These sections were then deparaffinized with xylene (#8587-4410, DAEJUNG, Gyeonggi-do, Korea), rehydrated with graded ethanol (decreasing concentrations of 100–70%), and finally washed with distilled water. The slides with liver sections were stained with hematoxylin (#MHS16, Sigma-Aldrich Co.) and eosin (#HT110332, Sigma-Aldrich Co.), washed with dH2O, and pathological changes were assessed using the Leica Application Suite (Leica Microsystems).
Liver tissue (50 mg) collected from each group was homogenized using PRO-PREP™ solution (#170841, iNtRON Biotechnology Inc., Sungnam, Korea), and total protein extracts were collected by centrifugation at 13,000 rpm for 5 min. The prepared proteins were subsequently subjected to 10% SDS-PAGE for 2 h at 100 V, and transferred to a nitrocellulose membrane (GE Healthcare, Little Chalfont, UK) for 2 h at 40 V in transfer buffer (25 mM Trizma-base, 192 mM glycine, and 20% methanol). Membranes were then exposed to appropriate dilutions of primary antibodies and allowed to hybridize overnight at 4℃: anti-IRE1αs antibody (#NB100-2324, Novus Biologicals, Littletone, Colorado, USA), anti-p-IRE1α antibody (#NB100-2323, Novus Biologicals), anti-CHOP antibody (#2895S, Cell Signaling Technology, Danvers, MA, USA), anti-eIF2α (#9722S, Cell Signaling Technology), anti-p-eIF2α (#9721S, Cell Signaling Technology), anti-Caspase-3 (#9662S, Cell Signaling Technology), anti-Bax antibody (#ab7977, abcam, Cambridge, UK), anti-Bcl-2 (#PA5-20068, Thermo, Massachusetts, USA) and anti-β-actin antibodies (#4967S, Cell Signaling Technology). After removal of the antibodies, the membranes were washed three times in a 10 mM Trizma-base (150 mM NaCl and 0.05% Tween-20) solution for 10 min. The membranes were subsequently incubated with horseradish peroxidase-conjugated anti-secondary antibody for 1 h at room temperature, after which they were washed again as described above, and developed using an enhanced chemiluminescence reagent plus kit (#DG-WF100, Dogen, Seoul,Korea). Finally, the results were quantified using the Image Analyzer System (VILBER Lourmat, Collégien, France) and expressed as the fold-increase over control values.
Statistical significance was evaluated using one-way analysis of variance (ANOVA) (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA) followed by Tukey's post hoc t-test for multiple comparisons. All values are reported as the means±SD, and P<0.05 was considered to indicate a statistically significant difference.
To investigate the improvement effects of EMfC on the fat accumulation in the liver of HFD-induced obesity mice, the area of accumulated fat was measured in the liver section stained with H&E. These levels were higher in HFD+Vehicle treated group than No treated group. However, a remarkable decrease of these areas was detected in HFD+OT and HFD+EMfC treated groups although decrease rate was greater in HFD+OT treated group (Figure 1). These results indicate that the fat accumulation can be successfully inhibits with EMfC treatment in HFD-induced obesity mice.
To examine whether anti-hepatic steatosis of EMfC can be accompanied with the subsequent abolition of the ER stress, an alteration in the levels of key proteins during the ER stress response were evaluated in the liver of HFD-induced obesity mice treated with EMfC. The alteration on the level of three key proteins was very similar in all groups. The level of eIF2α and IRE1α phosphorylation and CHOP expression were significantly increased after HFD treatment compared with No treated group. But, their levels were decreased after the treatment of OT and EMfC (Figure 2). Therefore, above results showed that EMfC with anti-hepatic steatosis activity can be successfully abolished the HFD-induced ER stress in the obesity mice model.
Apoptosis could be stimulated with the activation of prolonged unfolded protein response (UPR) [15]. To investigate whether the relive effect of EMfC for the ER stress can be accompanied with the subsequent abolition of the ER stress-induced apoptosis, an alteration in the expression level of apoptotic proteins were analyzed in HFD+EMfC treated mice. After HFD ingestion, an increase in the expression of three apoptotic proteins was observed in liver tissue. However, there was a significant decrease in the band density in the HFD+OT and HFD+EMfC treatment group when compared to the HFD+Vehicle treated group. Their decrease level was higher in HFD+OT treated group than HFD+EMfC treated group (Figure 3). These results suggest that the suppression effect of EMfC on the ER stress may be tightly correlated with the improvement of the ER stressinduced apoptosis.
ER stress and their sensors are tightly related with liver metabolism and the onset of hepatic steatosis because ER stress has been enhanced in the liver of obese animals and humans [161718]. Also, chronic hepatic ER stress was observed in the steatosis of ob/ob mice as well as mic with genetically modified PERK/eIF2α, IRE1/XBP1 and ATF6 signaling pathway [1920]. Based on above associated studies, the present studies especially focused on the applicability of this target in the hepatic steatosis during treatment with anti-obesity products. The results of the present study indicate first evidences that EMfC with anti-obesity effects can be inhibited the ER stress and ER stress-induced apoptosis in HFD-induced obesity models.
The significant suppression of various ER stress markers was reported in the liver and adipose tissue of HFD-induced model after the treatment of several compounds. The expression level of ATF6, p-PERK, p-IRE and p-JNK were remarkable decreased in the liver tissue of MLB treated obesity mice, while the similar patterns were detected on the expression level of XBP-1, CHOP, ATF4 and Bip in the liver tissue of HFD-induced obese model after the treatment of caffeic acid [89]. The expression level of IRE1, XBP, eIF2α and CHOP were significantly decreased in the liver tissue of HFD-induced rat model. In addition, adipose tissue of HFD-induced model showed similar results detected in the liver tissue. The treatment of taurine-conjugated derivative induced the decrease of GRP78 and CHOP expression in the adipose tissue of HFD-induced obesity C57BL/6 mice [10]. Meanwhile, the attenuation effects of mulberry leaf powder on the ER stress was investigated only in the GRP78 and their downstream markers. After the treatment of diet containing mulberry leaf powder, the expression level of these markers was remarkably decreased in EAM and DCM model [1213]. In the present study, we investigated whether the fermented products of mulberry leaves could improve the ER stress and ER stress-induced apoptosis in hepatic steatosis of HFD-induced obesity model. The alterations of several marker proteins for ER stress were significantly recovered after treatment with EMfC. Also, a similar decrease was detected on the expression of apoptosis protein as shown in Figure 3. These results are fully in agreement with previous results derived from HFD-induced model after the treatment of anti-ER stress compounds although the treated compounds and analysis items are different.
Meanwhile, OT used positive control in this study due to it was reported as new lipase inhibitor for the management of obesity. It suppresses absorption of dietary fat through inhibiting the activity of gastric and pancreatic lipases in the lumen of the gastrointestinal tract [21]. Also, the treatment of orlistat induces the improvement on the concentration of total cholesterol, LDL, glucose and insulin although some adverse effects including abdominal discomfort, liquid stools and flatulence were detected [2223]. Furthermore, it induces ER stress in tumor cells through enhancement of eIF2α phosphorylation, CHOP, ATF4 and GRP78 expression [24]. In the present study, the results about ER stress in HFD+OT treated group were differenced from those of previous study detecting in orlistat treated tumor cells. As showed Figure 2, the level of eIF2α and IRE1α phosphorylation, and CHOP expression were decreased in HFD+OT compared with HFD+Vehicle treated group. This difference on the effect of OT during ER stress response can be attributed to the physiological properties and disease condition of tumor cells and normal cells. In spite of these differences, the inhibition effects of orlistat to ER stress was greater than these of EMfC. These effects of orlistat were completely reflected on the fat accumulation and expression of apoptosis related proteins in the liver. However, more study are needed to understand what other factors determine the suppression of ER stress.
Taken together, the present study examined the suppression effects of EMfC on the ER stress reposes in the liver tissue of HFD-induced obesity model. The alteration of several key proteins in the ER stress response as well as ER stress-induced apoptosis were significantly improved in HFD+EMfC treated mice. Our results provide the first evidence that the anti-obesity effects of EMfC are tightly related with the improvement of ER stress response and their downstream pathway in the HFD-induced obesity model.
Figures and Tables
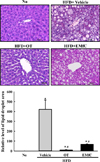 | Figure 1Measurement of fat accumulation in the liver tissue. (A) After the collection and staining of the liver tissue from the No, HFD+Vehicle, HFD+OT or HFD+EMfC treated group in C57BL/6 mice, their histological structure were observed at 400× magnifications using a light microscope. (B) Area of accumulated fat is presented as relative levels. Data represents the mean±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the HFD+Vehicle treated group. |
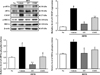 | Figure 2Expression of key marker proteins for ER stress. The expression levels of eIF2α, p-eIF2α, CHOP, p-IRE1α and IRE1α in total proteins were detected with specific antibodies. The level of β-actin is also shown as an endogenous control. The band intensity of these proteins was determined using an imaging densitometer, and the relative levels of each protein were calculated based on the intensity of actin protein as an endogenous control. Data represent the means±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the HFD+Vehicle treated group. |
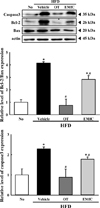 | Figure 3Expression of key marker proteins for ER stress-induced apoptosis. The expression levels of Caspase-3, Bcl-2 and Bax in total proteins were detected with specific antibodies. The level of β-actin is also shown as an endogenous control. The band intensity of the proteins was determined using an imaging densitometer, and the relative levels of each protein were calculated based on the intensity of actin protein as an endogenous control. Data represent the means±SD from three replicates. *P<0.05 compared to the No treated group. #P<0.05 compared to the HFD+Vehicle treated group. |
Acknowledgments
We thank Jin Hyang Hwang, the animal technician, for directing the Animal Facility and Care at the Laboratory Animal Resources Center. This research was supported by grants to Dr. Dae Youn Hwang from the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture and Forestry (116027-032-HD030).
References
1. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8(7):519–529.

2. Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010; 59(8):1899–1905.

4. Forman MS, Lee VM, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003; 26(8):407–410.

5. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.

6. Kim JE, Go J, Sung JE, Lee HA, Seo EJ, Yun WB, Hwang DY. Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats. Lab Anim Res. 2016; 32(1):16–23.
7. Achard CS, Laybutt DR. Lipid-induced endoplasmic reticulum stress in liver cells results in two distinct outcomes: adaptation with enhanced insulin signaling or insulin resistance. Endocrinology. 2012; 153(5):2164–2177.

8. Jeong JW, Lee B, Kim DH, Jeong HO, Moon KM, Kim MJ, Yokozawa T, Chung HY. Mechanism of action of magnesium lithospermate B against aging and obesity-induces ER stress, insulin resistance, and inflammsome formation in the liver. Molecules. 2018; 23(9):E2098.
9. Kim HM, Kim Y, Lee ES, Huh JH, Chung CH. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition. 2018; 55-56:63–70.

10. Chen Y, Wu Z, Zhao S, Xiang R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci Rep. 2016; 6:27486.

11. Seoane-Collazo P, Martínez de Morentin PB, Fernø J, Diéguez C, Nogueiras R, López M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014; 155(5):1679–1689.

12. Arumugam S, Thandavarayan RA, Veeraveedu PT, Ma M, Giridharan VV, Arozal W, Sari FR, Sukumaran V, Lakshmanan A, Soetikno V, Suzuki K, Kodama M, Watanabe K. Modulation of endoplasmic reticulum stress and cardiomyocyte apoptosis by mulberry leaf diet in experimental autoimmune myocarditis rats. J Clin Biochem Nutr. 2012; 50(2):139–144.

13. Arumugam S, Mito S, Thandavarayan RA, Giridharan VV, Pitchaimani V, Karuppagounder V, Harima M, Nomoto M, Suzuki K, Watanabe K. Mulberry leaf diet protects against progression of experimental autoimmune myocarditis to dilated cardiomyopathy via modulation of oxidative stress and MAPK-mediated apoptosis. Cardiovasc Ther. 2013; 31(6):352–362.

14. Lee MR, Kim JE, Yun WB, Choi JY, Park JJ, Kim HR, Song BR, Choi YW, Kim KM, Hwang DY. Lipolytic effect of novel extracts from mulberry (Morus alba) leaves fermented with Cordyceps militaris in the primary adipocytes derived from SD rats. Lab Anim Res. 2017; 33(3):270–279.
15. Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011; 31(12):2792–2797.

16. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306(5695):457–461.

17. Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008; 134(2):568–576.

18. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012; 18(1):59–68.

19. Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008; 7(6):520–532.
20. Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009; 119(5):1201–1215.

21. Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000; 20(3):270–279.

22. Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. J Hypertens. 1998; 16(12 Pt 2):2013–2017.

23. Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett SE, Kaplan RA, Comstock J, Lucas CP, Lodewick PA, Canovatchel W, Chung J, Hauptman J. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998; 21(8):1288–1294.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download