Abstract
Bacopa monnieri is a medicinal plant with a long history of use in Ayurveda, especially in the treatment of poor memory and cognitive deficits. In the present study, we hypothesized that Bacopa monnieri extract (BME) can improve memory via increased cell proliferation and neuroblast differentiation in the dentate gyrus. BME was administered to 7-week-old mice once a day for 4 weeks and a novel object recognition memory test was performed. Thereafter, the mice were euthanized followed by immunohistochemistry analysis for Ki67, doublecortin (DCX), and phosphorylated cAMP response element-binding protein (CREB), and western blot analysis of brain-derived neurotrophic factor (BDNF). BME-treated mice showed moderate increases in the exploration of new objects when compared with that of familiar objects, leading to a significant higher discrimination index compared with vehicle-treated mice. Ki67 and DCX immunohistochemistry showed a facilitation of cell proliferation and neuroblast differentiation following the administration of BME in the dentate gyrus. In addition, administration of BME significantly elevated the BDNF protein expression in the hippocampal dentate gyrus, and increased CREB phosphorylation in the dentate gyrus. These data suggest that BME improves novel object recognition by increasing the cell proliferation and neuroblast differentiation in the dentate gyrus, and this may be closely related to elevated levels of BDNF and CREB phosphorylation in the dentate gyrus.
The hippocampus is key limbic region that is involved in the formation of new memories [1]. Classically, the cholinergic system plays a role in the encoding of new memories; blockade of muscarinic cholinergic receptors via scopolamine impairs the encoding of new memories, but not the retrieval of previously stored memories [23]. In contrast, the drug-induced activation of nicotinic receptors enhances the encoding of new information [45]. Recent studies have demonstrated that the brain has regenerative potential throughout life in several regions, for example, the subgranular zone of dentate gyrus [67]. A recent study has demonstrated that adult neurogenesis in male rats plays a crucial role in the maintenance of hippocampal capacity for learning and memory formation, and enhancing hippocampal neurogenesis accelerates long-term potentiation decay and shows the rapid recovery of memory capacity [8]. Therefore, facilitation of hippocampal neurogenesis using various paradigms, including natural medicinal plants, may aid the rapid recovery of memory capacity and enhance memory functions.
Bacopa monnieri (L.), from the Scrophulariaceae family, is an aquatic plant found in Asian countries, including India. Bacopa monnieri has been used in Ayurvedic medicine to treat various diseases, including anxiety, poor memory, and cognitive deficits [9]. Several lines of evidence have demonstrated that Bacopa monnieri has a number of active components, such as alkaloids and saponin glycosides, including bacoside A [91011121314]. Bacopa monnieri extract (BME) enhances memory and cognitive functions via bacoside A [11]. In addition, administration of BME significantly increases the number of proliferating cells, as shown with 5-bromodeoxyuridine labeling, in chronic unpredictable stress (CUS)-induced depressed rats [15].
However, few morphological studies have measured the effects of BME on hippocampal neurogenesis in healthy animals and the underlying mechanisms. In the present study, therefore, we examined the role of BME on the novel object recognition, cell proliferation, and neuroblast differentiation in the dentate gyrus of healthy mice. In addition, we also observed changes in hippocampal brain-derived neurotrophic factor (BDNF) and phosphorylated cAMP response element-binding protein (CREB) as a possible mechanism of BME.
Male C57BL/6J mice (7 weeks of age) were purchased from Jackson Laboratory Co. Ltd (Bar Harbor, ME, USA). Mice were housed 5 per cage in a conventional area under standard conditions at ambient temperature (22±2℃) and humidity (60±5%), with a 12/12 h light/dark cycle with ad libitum access to food and water. Animal handling and care conformed to the guidelines of current international laws and policies (National Institutes of Health Guide for the Care and Use of Laboratory Animals, Publication No. 85-23, 1985, revised 1996) and were approved by the Institutional Animal Care and Use Committee of Seoul National University (Approval number: SNU-160816-21-1). All experiments were conducted with an effort to minimize the number of animals used, and the physiological stress caused by any procedures. All experimental procedures were conducted according to ARRIVE guidelines [16].
The mice were divided into three groups: control, vehicle (5% Tween 80)-treated, and BME-treated. Equal volumes of vehicle and BME (200 mg/kg) were administered to mice by oral gavage at 8 weeks of age, once a day for 4 weeks. This ensured that we could compare doublecortin (DCX) expression, which is exclusively expressed in immature neurons from 1 to 28 days of cell age, between groups [1718]. This dosage was adopted because several studies have demonstrated a cognitive-enhancing effect BME at this dosage [19].
The testing apparatus consisted of an open box (25×25×25 cm) made of black acryl, as previously described [20]. The floor was covered with woodchip bedding, which was moved around between trials and testing days to prevent the build-up of odor in certain places. The objects to be discriminated were made of solid metal and could not be displaced by the mice due to their weight. The objects were cleaned with bleach to remove residual odors.
On the 27th day of treatment with vehicle and BME, 2 h after treatment, mice from each group (n=10 per group) were allowed to explore the apparatus for 2 min. On testing day (28th day of treatment), two 2-min trials were performed 2 h following the last treatment. During the training trial, two identical objects were placed in opposite corners of the apparatus. Mice were placed singly in the apparatus and left to explore these objects. After training, mice were placed back in their home cage for an inter-trial interval of 1 h. Following this, one testing trial was performed, in which a new object replaced one of the familiar objects that was present in training trial. Mice were exposed again to the familiar and new object. Exploration was defined as directing the nose toward the object at a distance of no more than 2 cm and/or touching the object with the nose. From this measure, a series of variables were then calculated: the total time spent exploring the two identical objects in the training trial and the time spent exploring the two different objects in the testing trial.
The distinction between familiar and new objects in the testing trial was determined by comparing the time spent exploring familiar object with time spent exploring new object to create a discrimination index that represents the difference in exploration time expressed as a proportion of the total time spent exploring the two objects in testing trial.
Following the novel object recognition test, mice (n=5 in each group) were euthanized with 1.5 g/kg of urethane (Sigma-Aldrich, St. Louis, MO, USA) and perfused transcardially with 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4), as previously described [20]. The brains were removed and post-fixed for 12 h in the same fixative. The tissue was cryoprotected by overnight saturation with 30% sucrose. Serial brain sections were cut coronally at a thickness of 30 µm using a cryostat (Leica, Wetzlar, Germany), and collected in 6-well plates containing PBS until further processing.
All sections were processed under the same conditions to ensure that comparable immunohistochemical data between groups. Serial tissue sections, 90 µm apart, were selected from an area between 1.82 and 2.30 mm posterior to bregma, as defined using a standard mouse brain atlas [21]. Sections were sequentially treated with 0.3% H2O2 in PBS for 30 min and 10% normal goat serum in 0.05 M PBS for 30 min at 25℃. First, sections were incubated for 12 h with rabbit anti-Ki67 (1:1,000; Abcam, Cambridge, UK), rabbit anti-DCX (1:5,000; Abcam), or rabbit anti-pCREB (1:400; Cell Signaling Technology, Inc., Beverly, MA, USA) antibodies at 25℃. Thereafter, the sections were treated with biotinylated goat anti-rabbit IgG and a streptavidin-peroxidase complex (1:200; Vector, Burlingame, CA, USA) for 2 h at 25℃. Sections were visualized by reaction with 3,3-diaminobenzidine tetrachloride (Sigma) in 0.1M Tris-HCl buffer (pH 7.2) and mounted on gelatin-coated slides. Sections were dehydrated and mounted with Canada balsam (Kanto Chemical, Tokyo, Japan).
Analysis of DCX expression in the dentate gyrus was performed using an image analysis system and ImageJ v. 1.50 (National Institutes of Health, Bethesda, MD, USA). Data analysis was carried out under the same conditions by two observers for each experiment to ensure objectivity in blinded conditions, as described in a previous study [20]. Digital images of the whole dentate gyrus were captured with a BX51 light microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DP72, Olympus) connected to a computer monitor. Images were calibrated into an array of 512×512 pixels corresponding to a tissue area of 1200×900 µm (100×primary magnification). Each pixel had 256 gray levels and the intensity of DCX immunoreactivity was evaluated using relative optical density (ROD), which was obtained after transformation of the mean gray level using the following formula: ROD=log (256/mean gray level). The ROD of background staining was determined in unlabeled regions of sections using Photoshop CC 2018 software (Adobe Systems Inc., San Jose, CA, USA) and this value was subtracted to correct for nonspecific staining using ImageJ v. 1.50. Data are expressed as a percentage of the control group, which was set at 100%.
Ki67- and pCREB-immunoreactive nuclei in the whole dentate gyrus were counted using an analysis system equipped with a computer-based CCD camera (OPTIMAS software version 6.5; CyberMetrics® Corporation, Phoenix, AZ, USA; magnification, 100×), as previously described [20]. Images were converted to gray-scale, and Ki67- and pCREB-immunoreactive nuclei were automatically selected according to the intensity of the immunohistochemical staining for Ki67 and pCREB, respectively.
Following the novel object recognition test, mice in the control, vehicle-treated, and BME-treated groups (n=5 in each group) were sacrificed. Brain tissue was analyzed by western blotting, as described previously [20]. Briefly, the brain was removed and cut into 500-µm-thick sections on a vibratome (Leica Microsystems GmbH), and the hippocampal dentate gyrus was cut using a surgical blade. Hippocampal tissues were homogenized in 50 mM phosphate-buffered saline (PBS, pH 7.4) containing 0.1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylenediaminetetraacetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM sodium fluoride, 150 mM sodium chloride, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT). Following centrifugation for 5 min at 16,000 g at 4℃, the concentration of protein in the supernatant was determined using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Aliquots containing 20 µg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. Each aliquot was subsequently loaded onto a polyacrylamide gel. Following electrophoresis, the separated proteins in the gel were transferred to a nitrocellulose membrane (Pall Life Sciences, Port Washington, NY, USA). To reduce background staining, the membrane was incubated with 5% non-fat dry milk in PBS containing 0.1% Tween-20 for 45 min at 25℃, followed by incubation with rabbit anti-BDNF antibody (1:1000, Proteintech, Rosemont, IL, USA), peroxidase-conjugated goat anti-rabbit IgG (1:1000, Vector), and an ECL chemiluminescent reagent (Pierce; Thermo Fisher Scientific, Inc.).
The data were expressed as mean values in each group. To determine the changes in novel object recognition, cell number, and ROD, we compared the means between groups using one-way analyses of variance followed by Bonferroni's post-hoc test using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA, USA). Results were considered to be statistically significant if P<0.05.
During the training period, mice in all groups spent a similar amount of time exploring the two identical objects. During testing period, mice in all groups spent more time exploring the new object when compared with a familiar object. We found that BME-treated mice spent more time exploring the new object than the familiar one when compared with control or vehicle-treated mice; however, this did not reach statistical significance. There was no difference in discrimination index values between the control and vehicle-treated group; however, the mean discrimination index in the BME-treated group was significantly higher than the control or vehicle-treated groups (Figure 1).
In all groups, Ki67-positive proliferating cells were mainly detected in the subgranular zone of dentate gyrus, but there were significant differences in the number of Ki67-positive nuclei between groups. In the control group, the mean number of Ki67-positive nuclei was 11.44 per section, and there was a similar number of Ki67-positive proliferating cells in the vehicle-treated group. In contrast, we observed a large number of Ki67-positive proliferating nuclei in the dentate gyrus of BME-treated mice, compared with the control or vehicle-treated group. Furthermore, the number of Ki67-positive nuclei in the BME-treated group was 179.9% higher than that in the control group (Figure 2).
In all groups, DCX immunoreactive differentiated neuroblasts were found in the dentate gyrus with dendrites and somas. However, the number of somas and complexity of DCX-immunoreactive dendrites were significantly different between groups. Post-hoc analysis showed no difference in the number and ROD of DCX immunoreactive neuroblasts between the vehicle-treated group and control group. However, there was an increase in the number of DCX-immunoreactive differentiated neuroblasts with highly developed dendrites in the dentate gyrus of the BME-treated mice. The number of DCX immunoreactive neuroblasts and DCX immunoreactivity was significantly higher, by 149.0 and 159.0%, respectively, when compared with the control group (Figure 3).
pCREB-positive nuclei were found mainly in the subgranular zone of the dentate gyrus in all groups; however, there were significant differences in the number of pCREB-positive nuclei between groups. In the control group, the mean number of pCREB-positive nuclei was 70.48 per section, there was a reduction in the number of pCREB-positive nuclei in the vehicle-treated group compared with the control group; however, this did not reach statistical significance. Conversely, there was a significant increase in the number of pCREB-positive nuclei in the subgranular zone in the BME-treated group (149.7%) when compared with the control group (Figure 4).
BDNF levels in the dentate gyrus was slightly decreased in the vehicle-treated group compared to that in the control group. In the BME-treated group, BDNF levels were significantly increased in the hippocampal dentate gyrus homogenates by 167.9% of control group (Figure 5).
BME has various pharmacological actions, including antioxidant, anti-inflammatory, anticonvulsant, bronchodilator, and anti-ulcer effects [1022]. In addition, BME can affect the central nervous system, such as improving memory, reducing epilepsy and insomnia, and as an anti-anxiety agent [23]. In the present study, we observed a significant improvement of discrimination index in BME-treated healthy mice. This result is consistent with previous studies showing that BME improves cognitive function in type 2 diabetes [24], Alzheimer's disease [25], and amnesia [26]. In human trials, consumption of BME improves working memory and cognition in elderly individuals [142728]. An additional study showed that oral consumption of BME for 6 and 12 weeks increased information-retaining capacity over time; however, there was no beneficial effect on learning trials [27].
In the present study, we investigated cell proliferation and neuroblast differentiation in the dentate gyrus because the hippocampus is necessary for learning and memory retention, as shown in the Morris water maze task [29]. The present study shows morphological evidence that BME promoted cell proliferation and neuroblast differentiation in healthy mice. Chronic administration of BME increases the number of BrdU/NeuN-labled cells and DCX expression in the hippocampus of stressed rats [15]. However, this study found no significant differences in BrdU and/or DCX expression in the hippocampus of naïve (healthy) animals. Furthermore, an electrophysiological study has shown that BME enhances the magnitude of long-term potentiation with the inverted U-shaped dose-effect relationship in healthy animals [30].
To further elucidate the possible mechanisms underlying the BME-induced increases in cell proliferation and neuroblast differentiation, we focused on the BDNF-pCREB pathway. BDNF plays an important role in neurite growth, differentiation, and neuronal survival in the central nervous system. Mice lacking BDNF and its receptor, TrkB, showed a failure to respond to a behavioral regime [31]. In addition, the phosphorylation of CREB at Ser133 in neurons leads to the expression of neurotrophic genes, including BDNF, and regulates growth, survival, synaptic plasticity, short-term memory, and long-term potentiation [323334]. In addition, CREB participates in learning and memory through its involvement in adult hippocampal neurogenesis [35]. In the present study, we observed that BME administration significantly increased BDNF expression in the hippocampus. Previous studies have shown that administration of BME increases BDNF expression in the cerebrum of healthy and amnesic mice [34], and CUS-induced depressed rats [1536]. BME administration also increases the pCREB/total-CREB ratio in the hippocampus of amnesic mice [1237] and CUS-induced depressed rats [36]. In the present study, we found morphological evidence that BME administration significantly increased the number of pCREB-positive nuclei in the subgranular zone of the dentate gyrus. BME administration for 2 weeks increased new synaptic glutamate receptor proteins, such as N-methyl-D-aspartate receptor 2A/2B, and postsynaptic scaffolding proteins, such as postsynaptic density 95 [38]. Taken together, these data suggest that BME administration increases neural plasticity in the hippocampus.
In conclusion, the administration of BME promoted novel object recognition, and increased cell proliferation and neuroblast differentiation as well as upregulation of BDNF protein expression and CREB phosphorylation.
Figures and Tables
 | Figure 1Exploration time (n=10 per group) in training and testing trials, and discrimination index in the testing trial of familiar vs. new objects during the testing day of the novel object recognition test in control, vehicle-treated, and Bacopa monnieri extract (BME)-treated mice (n=10 per group; *P<0.05, vs. familiar object; aP<0.05, vs. control group; bP<0.05, vs. vehicle-treated group). All data are shown as % exploration time±SEM. |
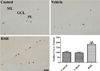 | Figure 2Ki67 immunohistochemistry in the dentate gyrus of control, vehicle-treated, and Bacopa monnieri extract (BME)-treated mice. Ki67-positive nuclei are most abundantly detected in the subgranular zone of dentate gyrus in BME-treated mice. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer. Scale bar=50 µm. The relative number of Ki67-positive nuclei in the dentate gyrus per section for each group are shown as a percentage of the value (n=5 per group; aP<0.05, vs. control group; bP<0.05, vs. vehicle-treated group). Data are presented as mean±SEM. |
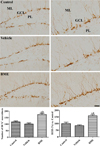 | Figure 3Immunohistochemistry for doublecortin (DCX) in the dentate gyrus of control, vehicle-treated, and Bacopa monnieri extract (BME)-treated mice. In the BME-treated group, DCX immunoreactive neuroblasts and their dendrites are most abundantly observed in the dentate gyrus. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer. Scale bar= 50 µm. The relative optical densities (RODs) expressed as a percentage of the value representing the DCX immunoreactivity in the dentate gyrus of the control group are shown. The number of DCX-immunoreactive neuroblasts in the dentate gyrus per section for each group are also shown (n=5 per group; aP<0.05, vs. control group; bP<0.05, vs. vehicle-treated group). Data are presented as mean±SEM. |
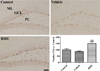 | Figure 4Immunohistochemistry for phosphorylated cAMP response element-binding protein (pCREB) in the dentate gyrus of control, vehicle-treated, and Bacopa monnieri extract (BME)-treated mice. Strong and abundant pCREB-positive nuclei are observed in the subgranular zone in BME-treated mice. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer. Scale bar=50 µm. The relative number of pCREB-positive nuclei in the dentate gyrus per section for each group are shown as a percentage of the value (n=5 per group; aP<0.05, vs. control group; bP<0.05, vs. vehicle-treated group). Data are presented as mean±SEM. |
Acknowledgments
This work was supported by a Basic Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1A2B6001941).
References
1. Khalaf-Nazzal R, Francis F. Hippocampal development-old and new findings. Neuroscience. 2013; 248:225–242.
2. Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav Neurosci. 2004; 118(1):223–236.

3. Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004; 145:207–231.

4. Buccafusco JJ, Letchworth SR, Bencherif M, Lippiello PM. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 2005; 26(7):352–360.

5. Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl). 2006; 184(3-4):523–539.

6. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998; 4(11):1313–1317.

7. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999; 2(3):260–265.

8. Alam MJ, Kitamura T, Saitoh Y, Ohkawa N, Kondo T, Inokuchi K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J Neurosci. 2018; 38(31):6854–6863.

9. Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013; 16(4):313–326.
10. Rastogi S, Pal R, Kulshreshtha DK. Bacoside A3--a triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994; 36(1):133–137.
11. Garai S, Mahato SB, Ohtani K, Yamasaki K. Bacopasaponin D--a pseudojujubogenin glycoside from Bacopa monniera. Phytochemistry. 1996; 43(2):447–449.
12. Saraf MK, Anand A, Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010; 35(2):279–287.
13. Liu X, Yue R, Zhang J, Shan L, Wang R, Zhang W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor Neurol Neurosci. 2013; 31(2):109–123.

14. Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012; 18(7):647–652.

15. Kumar S, Mondal AC. Neuroprotective, Neurotrophic and Anti-oxidative Role of Bacopa monnieri on CUS Induced Model of Depression in Rat. Neurochem Res. 2016; 41(11):3083–3094.

16. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8(6):e1000412.

17. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003; 467(1):1–10.

18. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21(1):1–14.

19. Rani A, Prasad S. A Special Extract of Bacopa monnieri (CDRI-08)-Restored Memory in CoCl2-Hypoxia Mimetic Mice Is Associated with Upregulation of Fmr-1 Gene Expression in Hippocampus. Evid Based Complement Alternat Med. 2015; 2015:347978.
20. Jung HY, Kim DW, Nam SM, Kim JW, Chung JY, Won MH, Seong JK, Yoon YS, Yoo DY, Hwang IK. Pyridoxine improves hippocampal cognitive function via increases of serotonin turnover and tyrosine hydroxylase, and its association with CB1 cannabinoid receptor-interacting protein and the CB1 cannabinoid receptor pathway. Biochim Biophys Acta Gen Subj. 2017; 1861(12):3142–3153.

21. Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;2001.
22. Sairam K, Rao CV, Babu MD, Goel RK. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine. 2001; 8(6):423–430.
23. Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006; 20(12):1023–1035.

24. Pandey SP, Singh HK, Prasad S. Alterations in Hippocampal Oxidative Stress, Expression of AMPA Receptor GluR2 Subunit and Associated Spatial Memory Loss by Bacopa monnieri Extract (CDRI-08) in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice. PLoS One. 2015; 10(7):e0131862.

25. Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer's Disease. Ann Neurosci. 2017; 24(2):111–122.
26. Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera Attenuates Scopolamine-Induced Impairment of Spatial Memory in Mice. Evid Based Complement Alternat Med. 2011; 2011:236186.
27. Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, Nathan PJ. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl). 2001; 156(4):481–484.
28. Peth-Nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhatkul N, Rangseekajee P, Ingkaninan K, Vittaya-Areekul S. Effects of 12-Week Bacopa monnieri Consumption on Attention, Cognitive Processing, Working Memory, and Functions of Both Cholinergic and Monoaminergic Systems in Healthy Elderly Volunteers. Evid Based Complement Alternat Med. 2012; 2012:606424.
29. Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999; 2(10):898–905.

30. Promsuban C, Limsuvan S, Akarasereenont P, Tilokskulchai K, Tapechum S, Pakaprot N. Bacopa monnieri extract enhances learning-dependent hippocampal long-term synaptic potentiation. Neuroreport. 2017; 28(16):1031–1035.
31. Lindholm JS, Castrén E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 2014; 8:143.

32. Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, Mamiya N, Okano E, Hasegawa S, Mercaldo V, Zhang Y, Maeda R, Ohta M, Josselyn SA, Zhuo M, Kida S. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011; 31(24):8786–8802.

33. Kida S, Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull. 2014; 105:17–24.

34. Konar A, Gautam A, Thakur MK. Bacopa monniera (CDRI-08) Upregulates the Expression of Neuronal and Glial Plasticity Markers in the Brain of Scopolamine Induced Amnesic Mice. Evid Based Complement Alternat Med. 2015; 2015:837012.
35. Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015; 8:46.

36. Hazra S, Kumar S, Saha GK, Mondal AC. Reversion of BDNF, Akt and CREB in Hippocampus of Chronic Unpredictable Stress Induced Rats: Effects of Phytochemical, Bacopa Monnieri. Psychiatry Investig. 2017; 14(1):74–80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download