Abstract
Stress severely disturbs physiological and mental homeostasis which includes adult neurogenesis in hippocampus. Neurogenesis in hippocampus is a key feature to adapt to environmental changes and highly regulated by multiple cellular signaling pathways. The primary cilium is a cellular organelle, which acts as a signaling center during development and neurogenesis in adult mice. However, it is not clear how the primary cilia are involved in the process of restraint (RST) stress response. Using a mouse model, we examined the role of primary cilia in repeated and acute RST stress response. Interestingly, RST stress increased the number of ciliated cells in the adult hippocampal dentate gyrus (DG). In our RST model, cell proliferation in the DG also increased in a time-dependent manner. Moreover, the analysis of ciliated cells in the hippocampal DG with cell type markers indicated that cells that were ciliated in response to acute RST stress are neurons. Taken together, these findings suggest that RST stress response is closely associated with an increase in the number of ciliated neurons and leads to an increase in cell proliferation.
In the adult brain, neurogenesis occurs in limited regions including the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus [1]. Neural stem cells residing in the SGZ regions undergo proliferation, differentiation, and maturation which result in generation of new functional and mature granule neurons. These processes are modulated by multiple external factors such as learning, exercise, and stress [2]. In addition, many genetic and molecular programs control neural stem cell maintenance and maturation processes. For example, hedgehog signaling in the SGZ region is responsible for the proliferation and maintenance of adult neural stem cells [3]. However, how distinct external factors affect adult neurogenesis at cellular and molecular levels is unclear.
Stress is a physical or psychological stimulus that triggers a particular biological response. As the duration or intensity of stress increases, several temporary and permanent changes are observed in the hippocampus. These changes include synaptic plasticity, morphological changes, and adult neurogenesis [4]. The responses of the endocrine, nervous, and immune systems to severe stimuli or stress factors, commonly known as stress responses, can affect homeostasis [5]. The hypothalamic-pituitary-adrenal (HPA) axis is a major component of the neuroendocrine system and is the most well-known stress response system. Ongoing stress leads to lasting activation of the HPA axis, resulting in mental disorders such as anxiety, insomnia, and depression [6].
The adult hippocampus shows remarkable structural and functional plasticity in response to external stimulus. Stress reduces the volume of the dentate gyrus while physical activity increases it through increased neurogenesis [7]. Repeated stress in the rat has been shown to result in a decrease in cell proliferation in the dentate gyrus [8]. Additionally, HPA axis activation by stress increases the release of glucocorticoids in humans and mice. Glucocorticoids are involved in the suppression of neurogenesis leading to the shrinkage of the dentate gyrus [9]. However, the exact role of glucocorticoids in hippocampal neurogenesis is controversial since during sexual experience, the high level of glucocorticoids was shown to increase neurogenesis [10].
A commonly used animla model of stress is the repeated restraint stress (RST), a modified form of immobilization stress. Inevitable physical and mental stress during RST is induced by placing an animal in a plastic tube to prevent movement. This is a well-established method of inducing stress and this type of stress has both physical and mental effects which occur simultaneously [11]. Restraint stress can cause several changes depending on the type of restraint stress and time in the tube, as reported in a recently published paper [12].
Primary cilia are non-motile cellular organelles protruding from the surface of most cells. They play a crucial role in transducing hedgehog signaling during development. The primary cilium is critical for the development of the vertebrate nervous system. It plays either essential or modulatory roles in specific neural developmental signaling pathways such as the hedgehog, Wnt, and platelet-derived growth factor (PDGF) signaling pathways [13]. Interestingly, recent studies indicate that the presence of primary cilia in adult neural stem cells in the DG is essential for the proliferation and establishment of adult neural stem cells in the hippocampus [1]. It is involved in Hedgehog signaling activation through the primary cilia on neural progenitor cells in the DG.
Here, we examined the role of primary cilia in regulating stress-induced adult neurogenesis in an acute RST mouse model. We observed an increase in the number of ciliated cells in the DG region due to repeated RST. Moreover, acute RST promotes cell proliferation in the hippocampus, and cell analysis indicated that these ciliated cells are neurons. These results suggest that during stress response in animals, ciliogenesis in neural stem cells may be regulated through adult neural stem cell maintenance or proliferation.
Adult male C57BL/6N mice (8 weeks old) were purchased from Orientbio Inc. (Seongnam, Korea). Mice were allowed to acclimate to the animal facility for at least one week before experiments were performed. Mice were given access to food and water ad libitum under standard conditions (22±2℃, humidity 40–60%) and a 12-h light-dark cycle (AM 9:00~PM 9:00). All animal experiments were approved by the Institutional Animal Ethical Committee of the Dongguk University prior to the start of the study.
Mice were subjected to restraint stress for 1, 3, 7, and 14 days using a ventilated 50 mL conical tube (SPL, Cat No. 50050) that allowed for a close fit to mice (2 hours/day between 10 a.m. and 12 p.m.). Control mice remained in the home cage until the behavioral experiment began.
Mice were anesthetized with CO2 and perfused with 1X phosphate buffered saline (PBS) followed by perfusion with 4% (w/v) paraformaldehyde (PFA). Collected brains were fixed overnight with 4% PFA and kept in 30% sucrose solution for 48 hours. After fixation, coronal sections (30 µm) of the brain samples were acquired. They were collected in 24-well plates (SPL) and stored at −20℃ in antifreeze solution until they were collected for staining.
For immunostaining, sections were treated with PBS three times for 10 minutes. The sections were rinsed and incubated for at least one hour in a blocking buffer (0.3% Triton X-100 and 0.2% gelatin in PBS). Tissues were incubated with primary antibodies overnight at 4℃, followed by incubation in secondary antibodies at 4℃ for two hours. The primary antibodies used were rabbit anti-AC (polyclonal) (1:1000; Santa Cruz), mouse anti-GFAP (1:500; Sigma), mouse anti-Nestin (1:400; Abcam), mouse anti-NeuN (1:500; Millipore), and rabbit anti-Ki67 (1:500; Abcam). The secondary antibodies used were conjugated to fluorescent dyes such as Alexa Fluor-488 and 594 (Jackson Immunoresearch). Images of stained tissues were obtained using a Nikon confocal laser scanning microscope. To analyze the fluorescently co-stained tissue samples, images were obtained after merging using the EZ-C1 software. Quantification of AC+ and Ki67+ cells in the DG of RST mice was performed in at least three independent sections using the ImageJ software.
The forced swim test was performed as described previously [14]. Mice were individually forced to swim by placing them in a cylinder (height: 45 cm and diameter: 20 cm) filled with water (25±1℃) to a height of 15 cm for six minutes. After 1 min of active swimming, the total duration of immobility was recorded during the last 5 min of the test. The immobility, swimming, and climbing behaviors were classified according to the movement of the mouse, and the immobility time was defined as the time when the mouse was completely immobile.
Statistical analysis for significance was performed with the one-tailed Student's t test for two different conditions, and comparisons between three or more conditions were performed with the one-way analysis of variance (ANOVA) and Tukey's multiple comparison test (GraphPrism). A P-value less than 0.05 was considered to be significant.
To examine changes in the primary cilia in the stress-affected dentate gyrus [1], we established an acute restraint stress mouse model. C57BL/6N mice were subjected to repeated restraint stress (RST) two hours a day for 1, 3, 7, and 14 days, and sacrificed 24 hours after the end of each experimental period (Figure 1A). Previous studies have shown that depression-like behavior occurs after chronic restraint stress [15]. Thus, we conducted behavioral experiments to confirm that the RST stress model we established was valid. We subjected mice to the forced swim test (FST), which is known to produce depression-like behavior in adult mice. Accordingly, the duration of immobilization was significantly increased to 205.29±10.57 s in the RST stress group compared to 165.83±14.55 s in the control group (CTRL: N=12, RST stress: N=14, P=0.0422) (Figure 1B). This result indicates that the RST stress animal model we established induces FST-associated depression-like behavior.
Stress is a known key factor which causes behavioral change and affects adult neurogenesis [16]. Recently, the primary cilia have been shown to play an important role in adult neurogenesis in the hippocampal dentate gyrus by increasing the number of postnatal hippocampal progenitors [17]. To examine the changes in primary cilia due to RST stress, we immunostained brain sections with the neuronal cilia specific antibody, adenylyl cyclase 3 (AC) (Figure 2). As a result, the frequency of cilia in the adult dentate gyrus regions after 3, 7, and 14 days of RST stress in the RST stress group was significantly increased compared to the control group (CTRL: 56.59±2.42% vs RST 3d: 72.37±2.07%, RST 7d: 73.31±2.51%, and RST 14d: 75.92±2.28%). No difference in the number of cilia was found after one day of RST stress between groups (8.75% of CTRL; 56.59±2.42% vs 61.54±2.76%, P=0.1973), as shown in Figure 1C–D. We also performed staining for ADP-ribosylation factor-like protein 13B (Arl13b), another ciliary marker gene, in RST mice. Interestingly, the cilia frequency increased significantly on RST day 1 (data not shown). It might be due to differences in the extent of interaction between ARL13B and ACIII antibodies and precursor or differentiating neurons. The ciliary length in the brain has also been shown to respond to physiological changes such as feeding [18]. We also measured the length of primary cilia in the DG region of the brains of mice which underwent RST but we did not observe any significant differences between the RST mice and control group (data not shown). These results suggest that ciliated cells in the hippocampal dentate gyrus region are affected by RST stress and that the extent to which they are affected might be correlated with the temporal programs of neural stem cell proliferation and differentiation.
We observed an increase in cilia frequency in the hippocampal DG in RST stress mice, as shown in Figure 1. It has been shown that primary cilia contribute to the increase in number of progenitor cells in the DG region. Additionally, stress downregulated the proliferation of neural stem cells in the DG. However, stress might promote adult neurogenesis in the hippocampus in certain conditions or species-specific cases [1019]. To evaluate how hippocampal neurogenesis is affected in our mouse model of stress, we examined adult neurogenesis in the RST stress condition. We immunofluorescently stained the differentiating neural precursor cells, NeuN+ cells, with the cell proliferation marker, Ki67, antibody. As a result, significantly more Ki67-positive cells were observed in the subgranular zone (SGZ) in the brains of mice who underwent acute RST (RST 1, 3 days) compared to the control group (RST 1 day: 35.71% of CTRL, P=0.0090, 3 days: 42.86% of CTRL, P=0.0026), as shown in Figure 2A–B. In contrast, there was no significant difference in the number of Ki67-positive cells observed in the subgranular zone of mice which underwent RST stress over an extended period of time (RST 7 days: 10.36% of CTRL, P=0.2303, 14 days: 1.25% of CTRL, P=0.9203) (Figure 3). These findings suggest that the increase in number of ciliated cells due to RST stress might be correlated with the upregulation of proliferating cells in the DG.
The SGZ, a region in the DG actively involved in adult neurogenesis, consists of several types of cells, including neural stem cells (NSCs). It regulates the proliferation, migration, and differentiation of neurons in this area, leading to adult neurogenesis [2021]. Our findings indicate that an increase in the frequency of primary cilia might be associated with adult hippocampal neurogenesis in acute RST stress mice. To investigate the role of primary cilia in early and late adult neurogenesis, we immunostained the primary cilia with various cell-specific markers including the NSC marker Glial fibrillary acidic protein (GFAP), the immature neuron marker beta-III-tubulin (Tuj1), and the mature neuronal marker NeuN (Figure 3A–B). The number of GFAP-positive NSCs with cilia significantly increased one day after undergoing RST stress (Figure 3B). An increase in the number of ciliated cells was also observed in Tuj1-positive immature neurons in brain samples one day after stress (Figure 3B). However, the number of mature neurons stained with the NeuN antibody did not significantly increase, as shown in Figure 3B. These results reveal that acute RST stress in mice affects only the number of ciliated cells among NSCs and immature neurons but not mature neurons. The increase in ciliogenesis in immature precursor cells in the DG might be important for cell maintenance and proliferation.
Primary cilia play important roles such as responding to changes in the environment and relaying various cellular signals in vertebrates. In the present study, we examined the role of primary cilia following RST stress in a mouse model. We have shown a possible association between primary cilia and neural stem cell proliferation during RST stress response. In the adult hippocampal DG of acute RST stress mice, the number of ciliated cells and Ki67-positive proliferating cells are increased in the early phase of stress response. However, the stress response does not induce an increase in the number of ciliated mature neurons. This suggests that the increase in ciliogenesis in the DG region after acute RST stress might regulate the increase in number of precursor cells in the adult hippocampal DG regions. Furthermore, primary cilia are important for stress response and neurogenesis in the mammalian system.
Primary cilia and Hh signaling are essential for adult stem cell formation and are known to play an important role in adult neurogenesis in the SGZ of the hippocampus [17]. Abnormality in or absence of primary cilia is associated with human diseases which manifest multiple clinical symptoms such as polydactyly, skeletal abnormalities, obesity, liver fibrosis, and polycystic kidney, termed “ciliopathies” [2223]. A sub-class of ciliopathies, the Bardet-Biedl syndrome (BBS), has defects in polydactyly, obesity, and mental retardation. In addition to these primary symptoms, BBS patients show neurological, speech, language, and behavioral abnormalities [23]. Moreover, it has been reported that BBS4 null mice (Bbs4-/-) become obese in a gender-dependent manner. Behavioral analysis of Bbs4-/- showed that mutant mice displayed an increase in anxiety/depression behaviors [24]. However, the exact role of primary cilia in adult neurogenesis in the hippocampal DG during stress response is unknown. Here, we observed an increase in the number of ciliated cells in the hippocampal DG of RST stress mice. Interestingly, the total number of ciliated ACIII-positive cells in the DG is gradually and significantly increased from day 3 to day 14 (Figure 2). However, the number of cells positive for another ciliary marker, Arl13b, which is more specific to non-neuronal cells or precursor cells, was significantly increased on day 1 after acute stress (data not shown). These results indicate that different cell types or cells in different phases of neurogenesis may be more ciliated. Our results also support the notion that in the nervous system, the primary cilia might sense and respond to physiological changes during feeding and stress. Interestingly, previous studies reported that ACIII-null mice showed no short-term memory and defect in contextual fear conditioning, which are related to the hippocampus [25].
Previous studies reported that various types of stresses, including RST stress, interfere with neurogenesis and cell proliferation in the hippocampus [2627]. In contrast, several lines of evidence from BrdU studies in acute RST-induced mice models showed an increase in cell proliferation in the DG and paraventricular nucleus (PVN) of mice subjected to acute RST stress [19]. Moreover, acute stress and neurogenesis studies in rat models reported that there was an increase in the proliferation of dorsal hippocampus neural precursor cells (NPCs) [28]. We confirmed the increase in cell proliferation in murine hippocampal DG due to acute RST stress (Figure 2). Although this controversy in cell proliferation during stress-related neurogenesis needs to be investigated further, our findings and previously reported studies suggest that the RST stress response might increase the proliferation of NPCs in the acute restraint stress mouse model.
Previous studies showed that the association between the primary cilia and SGZ neurogenesis is Hh signaling dependent [15]. GFAP+ neurons in Smo-deficient mice displayed defects in hippocampal neurogenesis [29], and the specific loss of ciliated genes such as Kif3a and IFT88 among NPCs results in defective hippocampal neurons due to premature cell-cycle exit [1730]. Similarly, postnatal deletion of cilia in neural stem/progenitor cells resulted in reduced numbers of proliferating doublecortin (DCX)-positive neuronal cells and amplifying progenitors (type 2a cells) without affecting radial NSCs [30]. Additionally, acute stress induces the expression of genes involved in neurogenesis and neuronal projection [31]. We observed that the cells which were ciliated in acute RST stress mice corresponded to immature progenitor cells positive for GFAP and Tuj1 markers. None of the ciliated cells were NeuN-positive differentiated neurons (Figure 3). Thus, these results suggest that acute RST stress might promote ciliogenesis among precursor cells in the SGZ and play an important role in neurogenesis during stress response.
In summary, RST stress increases cell proliferation and the number of ciliated cells in the adult hippocampal DG. These ciliated cells might participate in the proliferation of precursor cells of neural stem cells in response to stress. Although the physiological importance and molecular mechanism by which RST stress regulates ciliogenesis and neurogenesis are unclear, our study is the first to show that the primary cilia on neurons during the early phase of neurogenesis might be important during stress response.
Figures and Tables
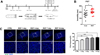 | Figure 1Restraint stress increases cilia frequency in the adult hippocampal dentate gyrus. (A) Experimental design for the RST stress. (B) Total immobility in the FST was measured for five minutes. The total immobilization time was significantly increased in mice subjected to restraint stress compared to control mice. (CTRL: 165.83±14.55 vs RST: 205.29±10.57, P=0.0422) (P<0.01 using the student's t-test) (C) Immunofluorescent staining for neuronal cilia with type 3 adenylate cyclase (AC) antibody and DAPI for nucleus (blue) in the left panel. An increase in ciliated cells stained with ACIII antibody was found in RST mice compared to control mice (CTRL). The cilia frequency in the hippocampal DG in RST stress mice was significantly increased 3, 7, and 14 days after RST stress. Quantification of primary cilia frequency. *P<0.01 using one-way ANOVA. |
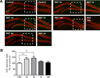 | Figure 2Cell proliferation in the hippocampal SGZ is upregulated by acute restraint stress. (A) To compare the extent of cell proliferation, sectioned brain samples were immunostained with Ki67 (green) and mature neuronal marker, NeuN, antibodies (red). (B) Quantification of Ki67-positive cells in acute RST stress mice and the control group. Although extended subjection to restraint stress did not significantly change the number of proliferating cells, RST stress upregulated the number of Ki67-positive cells in the SGZ. *P<0.01 using one-way ANOVA. |
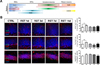 | Figure 3The number of ciliated neuronal progenitor cells is increased in acute restraint stress mice. (A) A schematic of different neuronal markers specific for the different stages of neurogenesis. (B) Immunostaining for AC (green), GFAP, Tuj1, and NeuN (red). For nucleus staining, DAPI (blue) was used. We found that the number of ciliated cells labeled with the immature neuronal cell markers, GFAP and Tuj1, increased in acute RST mice one day after stress, but that of mature NeuN-labeled neurons did not change. We observed that acute RST stress increased the cilia frequency among neurons during the early stage of differentiation in the hippocampal DG. *P<0.01 using one-way ANOVA. |
Acknowledgments
This research was supported by the Korea Mouse Phenotyping Project (NRF-2014M3A9D5A01073969) of the Ministry of Science and ICT (MSIT) through the National Research Foundation to H.W.K.
References
1. Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997; 21(6):801–810.

2. Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014; 94(4):991–1026.

3. Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003; 6(1):21–27.

4. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002; 3(6):453–462.

5. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006; 8(4):383–395.

6. Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007; 58:145–173.

7. Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PLoS One. 2009; 4(1):e4265.

8. Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003; 17(4):879–886.

9. Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994; 61(2):203–209.

10. Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010; 5(7):e11597.

11. Ko HW. The primary cilium as a multiple cellular signaling scaffold in development and disease. BMB Rep. 2012; 45(8):427–432.

12. Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009; 33(7):1089–1098.

13. Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009; 15(9):1062–1065.

14. Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977; 229(2):327–336.
15. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39(1):112–119.

17. Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008; 11(3):277–284.

18. Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, Kim HK, Gil SY, Yu JH, Lee B, Kim MS. Leptin-promoted cilia assembly is critical for normal energy balance. J Clin Invest. 2014; 124(5):2193–2197.

19. Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004; 368(1):7–10.

21. Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004; 478(4):359–378.

22. Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010; 11(5):331–344.

23. Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008; 85:225–260.
24. Eichers ER, Abd-El-Barr MM, Paylor R, Lewis RA, Bi W, Lin X, Meehan TP, Stockton DW, Wu SM, Lindsay E, Justice MJ, Beales PL, Katsanis N, Lupski JR. Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum Genet. 2006; 120(2):211–226.

25. Wang Z, Phan T, Storm DR. The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia. J Neurosci. 2011; 31(15):5557–5561.

26. Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009; 87(4):831–843.

27. Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009; 34(2):501–508.

28. Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013; 2:e00362.

29. Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003; 39(6):937–950.

30. Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011; 31(27):9933–9944.

31. Sannino G, Pasqualini L, Ricciardelli E, Montilla P, Soverchia L, Ruggeri B, Falcinelli S, Renzi A, Ludka C, Kirchner T, Grünewald TG, Ciccocioppo R, Ubaldi M, Hardiman G. Acute stress enhances the expression of neuroprotection- and neurogenesis-associated genes in the hippocampus of a mouse restraint model. Oncotarget. 2016; 7(8):8455–8465.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download