Abstract
Fitz-Hugh-Curtis syndrome has been described as perihepatitis associated with pelvic inflammatory disease (PID). It is classically seen in premenopausal young women who have right upper quadrant pain, usually but not always accompanied by symptoms of PID, and is frequently confused with biliary tract disease. However, the syndrome has rarely been reported in males. The predominant symptom is right upper quadrant pain, but PID may not be present in male patients. Here, we report a case of Fitz-Hugh-Curtis syndrome in a young male patient, which was diagnosed by serological tests and computed tomography. Fitz-Hugh-Curtis syndrome should be considered as a possible cause of pain in the right upper quadrant in male patients, although such a case is very rare.
Fitz-Hugh-Curtis (FHC) syndrome is perihepatitis without direct infiltration into the liver parenchyma in a patient with a pelvic inflammatory disease.1 In the 1930s, Curtis2 and Fitz-Hugh3 reported a syndrome characterized by violin string adhesions around the liver. Before the 1970s, only Neisseria gonorrhoeae (N. gonorrhoeae) was considered as a causative bacteria of FHC syndrome, but in 1978, Muller-Schoop et al.4 identified perihepatitis in a patient with an acute infectious disease using a laparoscope, suggesting Chlamydia trachomatis (C. trachomatis) is a new causative bacteria of FHC syndrome. In literature reported thereafter, C. trachomatis was identified in the cervix, urine tube, and hepatic capsule of a patient with perihepatitis.56 As the infection route from the pelvis to the liver, transmission through the peritoneal cavity is considered a major route and there were reports that transmission occurs through hematogenous infection or lymphatic vessels.78
Such a diagnosis was made in patients who had right upper abdominal pain and pelvic inflammatory disease, and from whom pathogens such as N. gonorrhea or C. Trachomatis were detected. For confirmatory diagnosis, there should be a finding of pelvic inflammation accompanying adhesions like violin strings between the hepatic capsule and the abdominal wall, and detection of strains from the adhered tissues should be performed.9 In consideration of the fact that the syndrome is a benign disease relatively easily treated with appropriate antibiotics, invasive surgeries are not performed except in the event of complications. Recently, diverse research has verified that the finding of perihepatic enhancement which was characteristic during the arteriographic phase of dynamic abdominal computed tomography was very specific to a diagnosis of FHC syndrome.810
FHC syndrome occurs in accompaniment with pelvic inflammatory disease, and as such, mostly women succumb to it and its occurrence in men is very rare. Looking at research performed in foreign countries, Kimbell and Knee11 reported FHC in a man for the first time in 1970, and FHC syndrome in men was reported by Francis and Osoba12 in 1972 and by Davidson and Hawkins et al.13 in 1982. However, only one case of FHC syndrome in a man14 has been reported in Korea. The authors diagnosed and treated one patient with FHC syndrome through dynamic abdominal computed tomography and blood and urine tests on sexually transmitted diseases, and therefore report this case.
A 23-year-old male patient visited our hospital with a main complaint of right upper abdominal pain that had occurred from one month before; he had no other past history with the exception of a history of vermiform process excision five years ago. In the right upper abdomen, there was continuous stinging and burning pain without improvement, and this pain was aggravated when he inhaled deeply or got up from a recumbent position. He was unmarried, and two months ago had had sex with a prostitute. There were no specific findings in his family history. According to his physical examination results, his height, weight, body mass index, blood pressure, pulse, respiration, and body temperature were 181 cm, 127 kg, 38.7 kg/m2, 150/90 mmHg, 108 beats/min, 20 breaths/min, and 36.2 degrees, respectively. His consciousness was clear. He had an acute ill-looking appearance and his physical examination results showed his abdomen was obese, his bowel sound was good and an enlarged organ or mass was not palpated, but there was tenderness in his right abdomen although it was smooth.
In his blood test conducted when he visited our hospital, his hemoglobin, white blood cells, and platelet levels were 17.3 g/dL, 9,580/mm3 (neutrophil: 63.2%, lymphocyte: 25.2%, monocyte: 6.6%, eosinocyte: 4.4%), and 208,000/mm3, respectively. In his serum biochemical test, his AST, ALT, total bilirubin, ALP, γ-GTP, serum urea nitrogen, and creatinine levels were 189 IU/L, 290 IU/L, 0.9 mg/dL, 265 IU/L, 75 IU/L, 12 mg/dL, and 0.89 mg/dL, respectively. His serum electrolyte was in a normal range. He tested negative for HAV IgG, HAV IgM, HBsAg, HBsAb, and HCV Ab. His CRP and procalcitonin levels were 0.25 mg/dL and 0.09 ng/mL, respectively, which were in normal ranges. There was no finding of abnormality in his urine test. Pathogens were not isolated in the blood and urine culture tests.
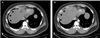
In electrocardiography, the rhythm was sinus rhythm, and in chest and abdominal simple X-ray tests, there was no finding of abnormality. In upper gastrointestinal endoscopy performed in order to exclude gastrointestinal diseases, there was no finding of abnormality. Due to suspicion of a biliary system disease like acute cholecystitis, abdominal ultrasonography and dynamic abdominal computed tomography were carried out. Except for a moderate level of fatty liver, there was no finding of abnormality in organs within the abdominal cavity including liver parenchyma and the pancreatico-biliary system, but thin linear capsular enhancement was noted at the front of the liver during arterial and lag phases (Fig. 1).
Based on the above test results, the possibility of perihepatitis was considered. Therefore, blood and urine tests for detecting a transmissible disease were additionally performed. Antibody and urine polymerase chain reaction tests for major causative bacteria, C. trachomatis, were conducted. Also, urine polymerase chain reaction tests for N. gonorrhea, Mycoplasma hominis (M. hominis), Mycoplasma genitalium (M. genitalium), Ureaplasmaurealyticum (U. urealyticum) were conducted. After conducting the above tests, he was administered with 100mg of doxycycline twice on the first day. Thereafter, it was decided to administer the agent once per day and ambulatory care was planned. On his visit one week later, his right upper abdominal pain had decreased. In additional tests on transmissible diseases, the result of his urine polymerase chain reaction test was positive for U. urealyticum, positive for C. trachomatis Ab IgG, and negative for IgM. He tested negative in the other tests.
It is known that FHC syndrome is perihepatitis accompanying five to 15% of patients with pelvic inflammatory disease, and largely occurs in young, sexually active women.1516 Its major infection route is direct transmission through inflammatory infection of the fallopian tube as a pelvic infection. In addition, transmission through the lymphatic vessels or the blood vessels is possible.8 Its major causative bacteria include C. trachomatis or N. gonorrhea, which may be identified through specimens obtained from the vagina, urine, and uterine cervix. It is known that its causative bacteria are detected most commonly from uterine cervix specimens. In the past, confirmative diagnosis was based on an observation of pelvic inflammation and the identification of strains from violin string adhesions and adhered tissues between the hepatic tissues and abdominal wall as a surgical method. However, in consideration of the fact that most often FHC syndrome is a benign disease that is relatively easily treated through appropriate antibiotic treatment, FHC syndrome may be diagnosed using an invasive method through a finding of perihepatic enhancement characteristic of dynamic abdominal computed tomography arteriography after clinical pathological tests following history taking and physical examination.11 Some researchers have observed findings of perihepatic enhancement during lag phase.8 There is no standardized antibiotic treatment for FHC syndrome, but like most treatments of acute pelvic inflammatory diseases, appropriate antibiotic treatment is applied. Oral antibiotics such as tetracycline, doxycycline, erythromycin, ofloxacin, or azithromycin have been used against major causative bacteria C. trachomatis.1718
In this case, there was no finding suggesting sepsis as an infection route. As a male patient, polymerase chain reaction was performed against sex mediated infectious causative bacteria and U. urealyticum was detected. It is estimated that transmission was made through the lymphatic vessels rather than being hematogenous transmission. Perihepatitis is considered to have occurred through urethritis. It is known that the major causative bacteria of urethritis include Chlamydia, Gonorrhea, and Mycoplasma. In addition, there was a finding of typical perihepatic enhancement during arteriographic phase and lag phase of dynamic abdominal computed tomography. FHC could be diagnosed by summing up history hearing, physical examination, findings by the laboratory and the characteristic finding of dynamic abdominal computed tomography. In this case, empirically, the patient was administered with doxycycline, the administration of which was maintained resulting from drug susceptibility in urine polymerase chain reaction test of U. urealyticum and non-steroidal anti-inflammatory drug (NSAID) was prescribed for pain.
FHC syndrome is not a rare disease in foreign countries, and there have been several reports of it domestically. However, it has occurred mainly to females.1920 The one case reported in 2010 is the only domestic case involving a male.14 Thus, the authors emphasize that when a cause is not certain based on a finding from the laboratory and chest and simple abdominal x-rays, the possibility of FHC syndrome in males as well as females should not be ignored, and active tests such as dynamic abdominal computed tomography and polymerase chain reaction test for transmissible diseases should be conducted.
Figures and Tables
References
3. Fitz-Hugh T. Acute gonococcic peritonitis of the right upper quadrant in women. JAMA. 1934; 102:2094–2096.

4. Müller-Schoop JW, Wang SP, Munzinger J, Schläpfer HU, Knoblauch M, Tammann RW. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. Br Med J. 1978; 1:1022–1024.

5. Wang SP, Eschenbach DA, Holmes KK, Wager G, Grayston JT. Chlamydia trachomatis infection in Fitz-Hugh-Curtis syndrome. Am J Obstet Gynecol. 1980; 138:1034–1038.

6. Lopez-Zeno JA, Keith LG, Berger GS. The Fitz-Hugh-Curtis syndrome revisited. changing perspectives after half a century. J Reprod Med. 1985; 30:567–582.
7. Counselman FL. An unusual presentation of Fitz-Hugh-Curtis syndrome. J Emerg Med. 1994; 12:167–170.

8. Nishie A, Yoshimitsu K, Irie H, Yoshitake T, Aibe H, Tajima T, et al. Fitz-Hugh-Curtis syndrome radiologic manifestation. J Comput Assist Tomogr. 2003; 27:786–791.
9. Ris HW. Perihepatitis (Fitz-Hugh--Curtis syndrome). A review and case presentation. J Adolesc Health Care. 1984; 5:272–276.
10. Joo SH, Kim MJ, Lim JS, Kim JH, Kim KW. CT diagnosis of Fitz-Hugh and Curtis syndrome: value of the arterial phase scan. Korean J Radiol. 2007; 8:40–47.

11. Kimball MW, Knee S. Gonococcal perihepatitis in a male. The Fitz-Hugh--Curtis syndrome. N Engl J Med. 1970; 282:1082–1084.
12. Francis TI, Osoba AO. Gonococcal hepatitis (Fitz-Hugh-Curtis syndrome) in a male patient. Br J Vener Dis. 1972; 48:187–188.
13. Davidson AC, Hawkins DA. Pleuritic pain: Fitz Hugh Curtis syndrome in a man. Br Med J (Clin Res Ed). 1982; 284:808.

14. Baek HC, Bae YS, Lee KJ, Kim DH, Bae SH, Kim DH, et al. [A case of Fitz-Hugh-Curtis syndrome in a male]. Korean J Gastroenterol. 2010; 55:203–207.

15. Lee SC, Nah BG, Kim HS, Choi TH, Lee SH, Lee JY, et al. [Two cases of Fitz-Hugh-Curtis syndrome in acute phase]. Korean J Gastroenterol. 2005; 45:137–142.
16. Owens S, Yeko TR, Bloy R, Maroulis GB. Laparoscopic treatment of painful perihepatic adhesions in Fitz-Hugh-Curtis syndrome. Obstet Gynecol. 1991; 78:542–543.
17. Oehme A, Musholt PB, Dreesbach K. Chlamydiae as pathogens--an overview of diagnostic techniques, clinical features, and therapy of human infections. Klin Wochenschr. 1991; 69:463–473.

18. Kechagia N, Bersimis S, Chatzipanagiotou S. Incidence and antimicrobial susceptibilities of genital mycoplasmas in out-patient women with clinical vaginitis in Athens, Greece. J Antimicrob Chemother. 2008; 62:122–125.

19. Ham YC, Lee KL, Shin DG, Kang Sk, Park SS, Yoon J, et al. Clinical Experience of Fitz-Hugh-Curtis Syndrome. J Korean Surg Soc. 2009; 76:36–42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download