Abstract
Endometriosis is an estrogen-dependent chronic inflammatory condition that affects women in their reproductive period and is associated with pelvic pain and infertility. Oxidative stress (OS) occurs when reactive oxygen stress (ROS) and anti-oxidants are in imbalance. OS is a potential factor involved in the pathophysiology of endometriosis. Iron-induced ROS may trigger a chain of events resulting in the development and progression of endometriosis. Endogenous ROS are correlated with increased cellular proliferation and ERK1/2 activation in human endometriotic cells. An oxidative environment leads to stimulation of the ERK and PI3K/AKT/mTOR signaling pathways that facilitate endometriotic lesion progression through adhesion, angiogenesis, and proliferation. OS is also known to be involved in epigenetic mechanisms in endometriosis. We summarize the recent knowledge in our understanding of the role of oxidative stress in the pathogenesis of endometriosis.
Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity. This estrogen-dependent chronic inflammatory condition affects women in their reproductive period and is associated with pelvic pain and infertility.1 The disease occurs in 5–10% in women at reproductive age, and it is 2–5 times more frequent in women with infertility.2 The most widely accepted hypothesis on the cause of this disease, first proposed by Sampson in 1927, is that viable endometrial tissue fragments move in a retrograde fashion through the fallopian tubes into the pelvic cavity during menstruation.3 There, these refluxed cells adhere, invade, and proliferate in the peritoneal cavity and form endometriotic lesions. The factors contributing to the establishment and persistence of endometriotic lesions most probably include abnormalities of the genital tract, genetic predisposition, hormonal imbalance, altered immune surveillance, inflammatory response, and abnormal regulation of the endometrial cells. Although numerous studies have been conducted, the pathogenesis of endometriosis remains unclear in reproductive medicine.
Oxidative stress (OS), characterized by an imbalance between pro-oxidant molecules including reactive oxygen and nitrogen species, and antioxidant defenses, resulting in damage to cellular lipids, proteins, or DNA, has been identified to play a damaging effects on female reproductive abilities. This imbalance between pro-oxidants and anti-oxidants can lead to a number of reproductive diseases such as endometriosis, polycystic ovary syndrome (PCOS), and unexplained infertility. Exposures to environmental pollutants are of increasing concern, as they too have been found to trigger oxidative states, possibly contributing to female infertility. In this review, we reviewed the basic knowledge of oxidative stress and then focused on the origin and role of oxidative stress in endometriosis.
ROS are generated during crucial process of oxygen (O2) consumption. They consist of free and non-free radical intermediates. As a diradical, O2 readily reacts with other radicals. Free radicals are often generated from O2 itself, and partially reduced species result from normal metabolic processes in the body. Reactive oxygen species are prominent and potentially toxic intermediates, which are commonly involved in OS. The Haber-Weiss reaction is the major mechanism by which the highly reactive hydroxyl radical is generated. Certain metallic cations, such as copper and iron may contribute to the generation of ROS.45
Physiological processes that use O2 creates large amounts of ROS, of which superoxide (SO) is the most common. Most ROS are produced in mitochondria and other sources includes endoplasmic reticulum (ER), cytochrome P450, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.6 ROS are formed as a natural byproduct of normal oxygen metabolism and have important roles in cell signaling and homeostasis. Anti-oxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), hemeoxygenase, and catalase exist. They neutralize excess ROS and prevent damage to cell structures. The SO anion is detoxified by superoxide dismutase (SOD) enzymes, which convert it to H2O2. Catalase and glutathione peroxidase (GPx) further degrade the end product to water. The antioxidant defense must be counterbalance the ROS concentration.
ROS are capable of reacting with other molecules to disrupt many cellular components and processes. The continuous production of ROS in excess can induce negative outcomes of many signaling processes. ROS do not always target the pathway. They also may produce abnormal outcomes by acting as second messengers in some intermediary reactions. Damage induced by ROS can occur through the modulation of cytokine expression and pro-inflammatory substrates by activation of redox-sensitive transcription factors AP-1, p53 and nuclear factor-kappa B (NF-kB). Under stable conditions, NF-kB remains inactive by inhibitory subunit I-kappa B. The increase of pro-inflammatory cytokines by interleukin (IL) 1-beta and tumor necrosis factor (TNF)-alpha activates the apoptotic cascade, causing cell death.7 The deleterious effects of excess ROS are opening of ion channels, lipid peroxidation, protein modifications and DNA oxidation.8
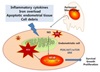
In various endocrine-related diseases, such as endometriosis, oxidative stress is increased.9 For these patients, erythrocytes, apoptotic endometrial tissue and cell debris in the peritoneal cavity by menstrual reflux and macrophages are potential inducers of oxidative stress.10 Pro-inflammatory cytokines may impact the recruitment of macorphages, which are one of the main producers of ROS.11 The peritoneal fluid is rich in lipoproteins, which generates oxidized lipid components in a macrophage-rich inflammatory environment. The oxidants exacerbate the growth of endometriosis by inducing chemo-attractants such as MCP-1 and endometrial cell growth-promoting activity. The presence of oxidative stress in the peritoneal cavity of women with endometriosis, the non-scavenging properties of macrophages that are non-adherent, and the synergistic interaction between macrophages, oxidative stress, and the endometrial cells.12 Signaling mediated by NF-κB stimulates inflammation, invasion, angiogenesis and cell proliferation. It may also inhibit the apoptosis of endometriotic cells. Overproduction of ROS impairs cellular function by altering gene expression via the regulation of redox-sensitive transcription factors such as NF-κB, which is implicated in endometriosis. NF-κB is activated in endometriotic lesions and peritoneal macrophages in endometriosis patients, which stimulates proinflammatory cytokine synthesis, generating a positive feedback loop in the NF-κB pathway. NF-κB-mediated gene transcription promotes a variety of processes, including endometriotic lesion establishment, maintenance, and development.13 Endometriotic cells have demonstrated relatively high ROS. These endogenous ROS are correlated with increased cellular proliferation and ERK1/2 activation in human.14
Iron-induced ROS may trigger a chain of events resulting in the development and progression of endometriosis. Iron overload was observed in the cellular and PF compartments of the peritoneal cavity of women with endometriosis.911 Iron mediated production of ROS via the Fenton reaction and induces OS. Iron overload-induces nitric oxide (NO) overproduction in apoptosis of peritoneal macrophages of women with endometriosis. Iron overload originated from retrograde menstruation or bleeding lesions in the ectopic endometrium, which may contribute to the development of endometriosis by a wide range of mechanisms, including oxidative damage and chronic inflammation. Macrophages also serve as the source of other inflammatory mediators contributing to the development of endometriosis. NO is a prime example, and when produced in abundance by NO synthase (iNOS, NOS2), induced by oxidant-sensitive transcription factors like NF-κB, has the potential to exacerbate endometriosis by promoting inflammation and necrosis at the site of lesion. Thus, excessive NO production is associated with impaired clearance of endometrial cells by macrophages, which promote cell growth in the peritoneal cavity.15 Endometriotic cysts contain high levels of free iron, due to. High concentrations of lipid peroxidation, DNA damage, and up-regulation of antioxidant system have been noticed. Long-standing history of the RBCs accumulated in the ovarian endometriotic cysts during the reproductive period produces oxidative stress that is a possible cause for the malignant change of the endometriotic cyst.16 An oxidative environment leads to stimulation of the ERK and PI3K/AKT/mTOR signaling pathways that facilitate endometriotic lesion progression through adhesion, angiogenesis, and proliferation.17 The suggested pathophysiology is summarized in figure 1.
It is evident that endometriotic cells contain high level of ROS. Impaired detoxification process lead to excess ROS and OS, and may be involved in increased cellular proliferation and inhibition of apoptosis in endometriotic cells. Investigating the mechanisms underlying oxidative stress associated with endometriosis may well prove useful for determining its specific pathways may be essential in future.
Endometriosis is an estrogen-dependent chronic inflammatory in women's reproductive period, and it is associated with pelvic pain and infertility. Oxidative stress (OS) occurs when reactive oxygen stress (ROS) and antioxidants are in imbalance, and it has been known to be a potential factor involve in the pathophysiology of endometriosis. This review well summarized the recent knowledge of the role of oxidative stress in the pathogenesis of endometrisois.
(Editorial Board)
References
1. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010; 362:2389–2398.
2. Eskenazi B, Warner ML. Epidemiology of Endometriosis. Obstet Gynecol Clin North Am. 1997; 24:235–258.

3. Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927; 3:93–110.43.
7. Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, et al. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007; 171:1168–1179.

8. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012; 10:49.

9. Carvalho LF, Samadder AN, Agarwal A, Fernandes LF, Abrão MS. Oxidative stress biomarkers in patients with endometriosis: Systematic review. Arch Gynecol Obstet. 2012; 286:1033–1040.

10. Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016; 106:1011–1017.

11. Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002; 77:861–870.

12. Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002; 955:183–198. discussion 19-200, 396-406.

13. Defrère S, González-Ramos R, Lousse JC, Colette S, Donnez O, Donnez J, et al. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. 2011; 26:1083–1092.
14. Ngô C, Chéreau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009; 175:225–234.

15. Pirdel L, Pirdel M. Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis. Reproduction. 2014; 147:R199–R207.

16. Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008; 14:32–40.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download