Abstract
Objectives
Chronic periodontitis is a common inflammatory disease of the oral cavity that causes destruction of periodontal tissues and bone around the teeth. Sclerostin is a protein encoded by the SOST gene. In this study, gingival crevicular fluid (GCF) levels of sclerostin in patients with chronic periodontitis were compared with those of healthy subjects.
Materials and Methods
In this case-control study, a total of 40 subjects were enrolled and divided into the healthy group (n=23) and chronic periodontitis group (n=17). GCF samples were collected, and the concentration of sclerostin was evaluated using enzyme-linked immunosorbent assay. Comparison of significance between groups was assessed using Mann-Whitney U test.
Chronic periodontitis is an inflammatory disease caused by interaction between microorganisms and the host immune system1. When the balance between bacterial virulence and the host immune system is disturbed, periodontal disease occurs, causing alveolar bone loss and periodontal destruction2. Microorganisms such as Tannerella forsythia and Porphyromonas gingivalis and their metabolites are the primary etiologic factors involved in the onset of periodontitis3. However, the disease is exacerbated by a series of endogenous agents, such as matrix metalloproteinases and inflammatory mediators including prostaglandin E2 and tumor necrosis factoralpha, resulting in activation of the bone resorption mechanism4.
The composition of gingival crevicular fluid (GCF) in periodontal diseases reflects the nature and extent of the host response to microbial plaques, and its evaluation provides quantitative assessment of biochemical markers for measuring cell metabolism5.
A newly cloned gene, SOST, encodes sclerostin, a protein that is a potent inhibitor of bone formation6. Sclerostin reduces the viability of osteoblasts and osteocytes and consequently leads to disturbances in bone turnover7. Sclerostin deficiency leads to sclerosteosis and Van Buchem disease, characterized by progressive bone thickening due to increased bone formation. This protein is produced by osteocytes8. Moreover, sclerostin is known as a marker of mature osteocytes and affects bone metabolism by inhibiting osteoblast proliferation and differentiation910.
Considering the potential role of sclerostin in bone metabolism, and because only one study evaluated GCF level of sclerostin in patients with periodontitis, in this study, the GCF levels of sclerostin in patients with chronic periodontitis and healthy individuals were compared.
In this case-control, cross-sectional study, 40 patients, 22 males and 18 females, between 25 and 50 years of age were enrolled from 2016 to 2017 at Department Periodontics, Shahid Beheshti University of Medical Sciences (Tehran, Iran). All subjects provided written informed consent, and the institutional ethics review committee of Shahid Beheshti University of Medical Sciences approved this study (approval no. IR.SBMU.RIDS.REC.1395.335). The subjects were divided into two groups: healthy group (n=23) and chronic periodontitis group (n=17). Criteria for healthy subjects were gingival index <1, pocket depth (PD) <3 mm, and no clinical attachment loss (CAL). Patients with chronic periodontitis were selected according to the American Academy of Periodontology (AAP) criteria 1999, having at least two teeth with PD ≥5 mm and CAL ≥4 mm with bleeding on probing at the affected sites. Exclusion criteria were smoking, systemic disease (i.e., diabetes mellitus, rheumatoid arthritis, and systemic bacterial, fungal or viral infection), pregnancy, or history of drug therapy or periodontal therapy during the past 6 months. Periodontal examination was performed by the same periodontist.
The sampled teeth were isolated with cotton and air dried. Supragingival plaque was removed carefully with a sterile scaler. GCF samples were collected from two locations, with the deepest PD achieved by placing paper points (#25) using the intrasulcular method. Samples were ensured to be free of saliva or blood and immediately transferred to sterile microtubes containing 250 µL phosphate-buffered saline. All samples were centrifuged at 4℃ and 3,000g for 10 minutes. The supernatant was collected and immediately frozen at −70℃ until subsequent analysis.
The total concentration of sclerostin was measured using a commercially available enzyme-linked immunosorbent assay kit (Thermo Fisher Scientific, Waltham, MA, USA). All procedures were performed in duplicate according to the manufacturer's instructions. Optical densities at 450 nm were measured (reference wavelength 570 nm). Next, sclerostin concentration in the GCF was determined by comparing the average absorbance readings of each sample with the concentrations in the assay standard curve.
Statistical analysis was conducted using IBM SPSS Statistics software (ver. 21; IBM Co., Armonk, NY, USA). In addition, Mann-Whitney U test was used to examine the relationship between sclerostin levels in the patient and healthy groups. P<0.005 was considered statistically significant. Data are presented as the mean±standard deviation.
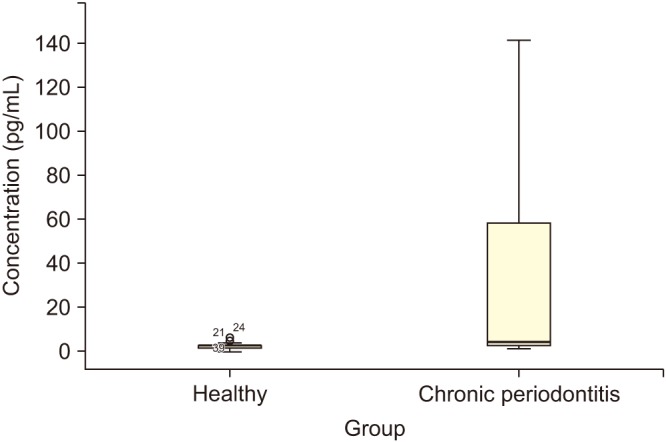
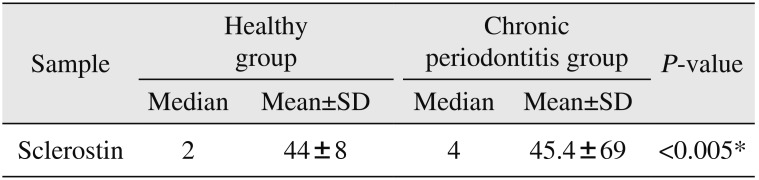
Median and mean values of GCF concentration of sclerostin in both healthy and patient groups are outlined in Table 1 and Fig. 1. Statistical results showed a significant difference in the concentration of sclerostin in patients with chronic periodontitis compared with the healthy subjects (P<0.005).
Chronic periodontitis is a common inflammatory disease of the oral cavity resulting in destruction of periodontal tissues and bone surrounding the teeth. To date, many studies have been conducted on the assessment of inflammatory status caused by interaction of the host immune system and cytokines and interleukins in the development of chronic periodontitis11. However, only a few studies have focused on the role of GCF proteins such as sclerostin and their effects on bone metabolism in vivo and in vitro.
Since the physiology of bone metabolism is based on a pivotal equilibrium between osteoblasts and osteoclast cells, the focus of most studies is the axial relationship of these cells and their direct function on formation, density, and volume of bone in the body1213, with less attention to GCF proteins and their role in diagnosis and prognosis of periodontal disease.
GCF composition reflects the periodontal inflammatory status as a result of the interplay between the bacterial biofilm and periodontal tissues. Collection of GCF could be a good alternative to invasive diagnostic methods, including serum samples or gingival biopsies5. GCF assessment is quick, easy to perform, feasible at all tooth sites, and, most importantly, produces no or little trauma to gingival tissues14.
The results of this study showed the mean GCF level of sclerostin in patients with chronic periodontitis was significantly higher than that in healthy subjects (P<0.005) and is in agreement with a similar study in which the sclerostin level and the ratio of receptor activator of nuclear factor-κB ligand (RANKL) to osteoprotegerin (OPG) in GCF of periodontal diseases were examined; the GCF level of sclerostin may be more reliable than the RANKL/OPG ratio as a diagnostic and prognostic marker of periodontal disease and treatment outcome12.
In most studies, chronic periodontitis phenotype and bone resorption were associated with discovery of the SOST gene11. Administration of sclerostin antibody in ovariectomized mice and monkeys resulted in dose-dependent increases in bone volume and density, as well as bone formation on trabecular, periosteal, and endosteal surfaces1516. Sclerostin may be linked with periodontal disease and is potentially a strong candidate for bone protection and an effective therapeutic target for treatment of periodontal diseases. Therefore, it may be possible to use different strategies and therapeutic approaches in the near future to control periodontal disease and prevent bone destruction.
In studies conducted using placebo and single dose antisclerostin antibodies, a potential therapeutic benefit was observed in patients with bone resorption and those with rheumatoid arthritis17. Therefore, anti-sclerostin antibodies could prevent bone resorption in the body and, in bone marrow spaces, were associated with bone formation and increased differentiation of osteoblasts18. Conversely, deletion of the sclerostin gene inhibits the Wnt/β-catenin signaling pathway and results in positive and constructive signals for PDL fiber reconstruction, which can be attributed directly to the presence of Sharpey fibers1117.
Regarding the complex interaction of the host immune system, including cytokines, interleukins, and bacterial agents, it is difficult to understand the role of sclerostin in the extent and severity of periodontal disease. Additional studies are required to better understand the roll of sclerostin in periodontal disease.
Acknowledgements
This study was supported by a research grant from School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This paper is based on a thesis submitted in partial fulfillment of the requirements for the Degree of Dentistry at International Branch, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Notes
Authors' Contributions: S.K.V. participated in data collection and wrote the manuscript. Z.R.E. participated in the study design and revised the manuscript. Z.Y. and M.K. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997; 14:33–53. PMID: 9567965.

2. Bascones-Martínez A, Muñoz-Corcuera M, Noronha S, Mota P, Bascones-Ilundain C, Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: basic mechanisms. Med Oral Patol Oral Cir Bucal. 2009; 14:e680–e685. PMID: 19680192.
3. Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003; 82:338–344. PMID: 12709498.

4. Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011; 38(Suppl 11):85–105. PMID: 21323706.

5. Champagne CM, Buchanan W, Reddy MS, Preisser JS, Beck JD, Offenbacher S. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol 2000. 2003; 31:167–180. PMID: 12657001.

6. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008; 283:5866–5875. PMID: 18089564.

7. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003; 22:6267–6276. PMID: 14633986.

9. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005; 19:1842–1844. PMID: 16123173.

10. Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, et al. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011; 26:1425–1436. PMID: 21312267.

11. Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, et al. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J. 2015; 29:2702–2711. PMID: 25757567.

12. Balli U, Aydogdu A, Dede FO, Turer CC, Guven B. Gingival crevicular fluid levels of sclerostin, osteoprotegerin, and receptor activator of nuclear factor-κB ligand in periodontitis. J Periodontol. 2015; 86:1396–1404. PMID: 26367496.

13. Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008; 23:860–869. PMID: 18269310.

14. Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003; 31:32–42. PMID: 12656994.

15. Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009; 24:578–588. PMID: 19049336.

16. Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010; 25:948–959. PMID: 20200929.

17. Yang X, Han X, Shu R, Jiang F, Xu L, Xue C, et al. Effect of sclerostin removal in vivo on experimental periodontitis in mice. J Oral Sci. 2016; 58:271–276. PMID: 27349550.
18. Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV, et al. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res. 2013; 28:2347–2356. PMID: 23712325.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download