Abstract
Pneumatocele (PC) is a thin-walled cyst of the lung that can occur at all ages and with various etiologies. However, there is no fully accepted consensus for the management of PC in a neonatal intensive care unit. Although the management of PC is generally expectant, it is difficult to decide how long conservative management should be maintained, especially under Korea's medical care environment and the parents' worry and anxiety. We report a male neonate, born at 27+5 weeks gestation, weighing 1,000 g, who had a post infectious PC caused by methicillin-resistant Staphylococcus aureus sepsis. We treated conservatively for about 100 days (roughly 14 weeks), but unfortunately after a few days of chest retraction, acute exacerbation occurred, video assisted thoracoscopic surgery (VATS) was deemed necessary and performed. The purpose of this publication is to describe the clinical course, aggravation and relief after VATS management with a review of the literature.
Pneumatocele (PC) is a thin-walled cyst of the lung that can occur at all ages and with various etiologies.12 During the 1970s and 1980s, the most common cause of PC in neonates was related to the use of a ventilator, however, as the use of the surfactant increased, the incidence of PC in neonates has declined.2 Post-infectious PC is also seen in neonates secondary to severe pulmonary infection.3 Importantly, there is no fully accepted consensus for the management of PC in a neonatal intensive care unit (NICU). In 2005, Imamoğlu et al.4 studied the records of 58 children treated for postpneumonic empyema with PC and suggested a postpneumonic PC treatment algorithm. However, there are some limitations when attempting to apply the algorithm to preterm neonates in a NICU. The purpose of this publication is to describe the clinical course, aggravation and relief after video assisted thoracoscopic surgery (VATS) management of necrotizing PC in mechanically ventilated very low birth weight infant (VLBWI).
A male neonate, born at 27+5 weeks gestation, weighing 1,000 g was 1st baby of twin male neonates. The Apgar scores were 3 and 6 at 1, 5 minutes and the patient was intubated and given one dose of surfactant in the delivery room. From day of life (DOL) 1 to 6, ampicillin and gentamicin were used in an attempt to treat probable neonatal early onset sepsis. During DOL 1–8, the patient received ventilator care with synchronized intermittent mandatory ventilation (SIMV) mode targeted oxygen saturation 88–93% with fraction of inspired oxygen (FiO2) 0.21, on DOL 8, high-frequency oscillation ventilation (HFO) care started with FiO2 0.3. On DOL 11, due to repeated desaturation, SIMV care support was initiated and blood samplings (including blood culture) were performed. On DOL 11, C-reactive protein level (reference range, <0.5 mg/dL) was significantly elevated to 20.8 mg/dL and the presence of methicillin resistant Staphylococcus aureus (MRSA) was identified in the blood culture test. Chest X-ray showed patchy consolidation on both lower lobes, associated possible pneumonia, underlying hyaline membrane disease. Immediately, vancomycin and imipenem were initiated. After that, MRSA cultured in sputum endotracheal aspirate, though blood culture results did not confirm the growth. Sepsis and disseminated intravascular coagulopathy occurred sequentially, we intervened appropriately (including inotropics and several transfusions).
On DOL 14, a chest X-ray showed significant diffuse reticulonodular and ground glass opacities on both lungs, however, on DOL 15, a chest X-ray revealed a cavitary lesion (probable PC) in left lower lung field (Fig. 1). At that time, venous blood gases was stable: pH 7.420, pCO2 32.8 mmHg, pO2 30.0 mmHg, HCO3− 21.3 mmol/L, and base excess −3 mmol/L in SIMV care support with FiO2 0.21–0.3. Target O2 saturation was changed from 88–93% to 90–95%. Desaturation occurred several times (average, 5–10 times/day) and disappeared rapidly after FiO2 0.6 support.
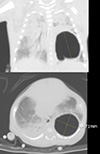
On DOL 53, a chest computed tomography was performed due to the increasing size of the PC and demonstrated a single, large PC max 3×2.9×3.6 cm size cystic lesion in the left lower lobe with several internal septation, mild irregular, rather thick wall. Also, peribronchial, perihilar increased opacity with ground glass opacity in the both lung and atelectasis in the dependant portions of both lungs (Fig. 2). Because of his stable clinical condition and a decreasing frequency of desaturation (average, 1–5 times/day) with FiO2 0.21–0.3, we decided to observe closely despite the increasing size of the PC. Additionally, the patient received a 10 days course of dexamethasone treatment to prevent the incidence of bronchopulmonary dysplasia. From DOL 59 to DOL 119, the patient had non-invasive positive ventilation (NIV) care including biphasic positive airway pressure, high flow nasal cannula and nasal prong.
On DOL 121, after a few days of chest retraction, acute exacerbation occurred, 40% of desaturation regardless of ambu bagging, leading to the re-intubation and SIMV. As the PC did not seem to resolve, sono guided aspiration of the PC was attempted without success. Additionally, his condition did not improve and the PC did not respond to the intercostal chest drain (ICD). The next day, on DOL 122, though his body weight was 3.6 kg, we performed the VATS management and general condition was successfully improved. The specimen received from the operation room was determined to be a cystic lesion, measuring 4.5×3.0×1.0 cm in size PC, consistent with acute alveolar hemorrhage. The cavitary lesion was communicated to the bronchus.
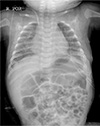
On DOL 126, the patient was weaned from ventilator care and his clinical condition significantly improved. On DOL 133, the patient was discharged following a normal chest radiograph and resolution of the PC (Fig. 3).
PC is a thin walled cyst of the lung that is frequently observed in the NICU. Unfortunately, there are no definite guidelines for conservative management versus invasive management of PC. It is difficult to decide how long conservative management should be maintained, especially under Korea's medical care environment and the parents' worry and anxiety.
In 1995, Joosten et al.5 reported 36 cases of the staphylococcal pneumonia and lower mortality due to early surgical intervention. They suggested guidelines recommending early thoracotomy in young children, especially under 1 year old. They suggest that the early detection and treatment of pulmonary complications (such as PC, pleural fluid, pneumothorax, etc.) with thoracentesis and/or chest tube drainage are important. Furthermore, if the radiological features of disease do not improve (though they did not mention the period), thoracotomy should be considered. In addition, Arora et al.3 in a retrospective NICU chart review of 27 PC infants, reported positive endotracheal culture (though the organism was variable, MRSA was the most common organism) correlated with persistence of PC, that was associated with a higher mortality. Therefore, as in our case, in the NICU, especially post-infectious PC in VLBWI, early intervention might be lead to a decrease length of hospitalization and improve patient relief.
After being proposed an algorithmic approach of PC in postpneumonic empyema in 2005,4 many case reports had been published to support the evaluation of the algorithm. Joseph et al.6 published a case report of conservative treatment in a large post-infectious PC. Though their patient had a complicated PC according to the algorithm, invasive management was indicated and they opted to closely observe in the pediatric intensive care unit and discharged on DOL 33. However, in the discussion section, they expressed concern about a possibility of abrupt tension formations, rupture of the PC into the pleural space and occurrence of tension pneumothorax.
In 2015, Al-Ghafri et al.7 published two cases of PC in infants with ventilator care, one treated conservatively with HFO. As previously described, HFO decreases airway pressures, reduces the risk of barotrauma, and improves gas exchange. After one week of HFO support, the PC resolved. The other patient treated with HFO for three weeks, failed to wean from HFO four times, and treated with ICD. The authors suggested that conservative management treatment should be performed as long as the patient's condition allows.
In our case, we treated conservatively for about 100 days (roughly 14 weeks) and brief clinical course and especially pCO2 levels are shown in Fig. 4. One thing to note is that the pCO2 level is kept relatively higher (pCO2 >60 mmHg) than the average during the NIV period (DOL 59–119). During the NIV period, the patient was clinically stable, desaturation appeared 1–5 times a day, there was rarely the need for O2, and we maintained close observation in NICU. However, on DOL 122, surgical treatment, VATS management was deemed necessary and performed.
In 2014, Sacks et al.8 reported surgical salvage of PC with unresponsive medical treatment in extremely low birth weight infant. In addition, they note that the risk of injury either lung cysts or healthy lung parenchyma during the period of chest tube placement. So when there is a potential need for surgical salvage, early surgical consultation should be considered.
There are many non-surgical options of conservative management when a PC appears in NICU. Lateral decubitus positioning with the affected side down, selective intubation on the uninvolved side, HFO can be easily tried first. If there is no change in this way, chest tube insertion, sono guided aspiration, fibrin sealant injection via pigtail catheter can be attempted in a less invasive way.9 If every attempts fail, surgical management is recommended.
In recent years, minimal invasive surgical techniques (MIS) are used to perform operative procedures avoiding the morbidity and mortality of open surgical wounds. As VATS is one of the MIS, among babies <5 kg weight, quite a significant number of patients are treated successfully. With the development of MIS in neonates over the recent years, its contraindications in its use have become relative rather than absolute. However, not all the babies are good candidates for thoracoscopic surgery and more studies are required to set up the criteria.1011
In conclusion, as suggested by many literatures, expectant management should be the primary options in the occurrence of the PC. However, as in our case, the PC appeared secondary to an infection is correlated with persistent and relatively poor prognosis. Also, high level of pCO2 can be one of the predictive factors of poor prognosis. More research is needed to better establish the expectant period and treatment of the PC in NICU especially preterm infants.
Figures and Tables
Fig. 1
Chest X-ray on DOL 15. A cavitary lesion in LLLF, suggested PC was identified with SIMV care with FiO2 0.3. DOL, day of life; LLLF, left lower lung field; PC, pneumatocele; SIMV, synchronized intermittent mandatory ventilation.
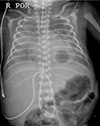
Fig. 2
CT images on DOL 53. A single, large PC was identified and the size was larger than the previous examination. CT, computed tomography; DOL, day of life; PC, pneumatocele.
References
1. Quigley MJ, Fraser RS. Pulmonary pneumatocele: pathology and pathogenesis. AJR Am J Roentgenol. 1988; 150:1275–1277.

2. Hussain N, Noce T, Sharma P, Jagjivan B, Hedge P, Pappagallo M, et al. Pneumatoceles in preterm infants-incidence and outcome in the post-surfactant era. J Perinatol. 2010; 30:330–336.

3. Arora P, Kalra VK, Natarajan G. Pneumatoceles in infants in the neonatal intensive care unit: clinical characteristics and outcomes. Am J Perinatol. 2013; 30:689–694.
4. Imamoğlu M, Cay A, Koşucu P, Ozdemir O, Cobanoğlu U, Orhan F, et al. Pneumatoceles in postpneumonic empyema: an algorithmic approach. J Pediatr Surg. 2005; 40:1111–1117.
5. Joosten KF, Hazelzet JA, Tiddens HA, Hazebroek FW, Dzoljic-Danilovic G, Neijens HJ, et al. Staphylococcal pneumonia in childhood: will early surgical intervention lower mortality? Pediatr Pulmonol. 1995; 20:83–88.

6. Joseph L, Shahroor S, Fisher D, Goldberg S, Picard E. Conservative treatment of a large post-infectious pneumatocele. Pediatr Int. 2010; 52:841–843.

7. Al-Ghafri M, Al-Hanshi S, Al-Ismaily S. Two cases of pneumatoceles in mechanically ventilated infants. Oman Med J. 2015; 30:299–302.

8. Sacks GD, Chung K, Jamil K, Garg M, Dunn JC, DeUgarte DA. Surgical salvage of acquired lung lesions in extremely premature infants. Pediatr Surg Int. 2014; 30:573–576.

9. Park TH, Kim JK. Nonsurgical management of an enlarging pneumatocele by fibrin sealant injection via pigtail catheter. Pediatr Pulmonol. 2016; 51:E5–E7.

10. Rothenberg SS, Middlesworth W, Kadennhe-Chiweshe A, Aspelund G, Kuenzler K, Cowles R, et al. Two decades of experience with thoracoscopic lobectomy in infants and children: standardizing techniques for advanced thoracoscopic surgery. J Laparoendosc Adv Surg Tech A. 2015; 25:423–428.

11. Mitul AR, Sarin YK. Minimal access surgery in neonates. J Neonatal Surg. 2017; 6:59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download