Abstract
Background and Purpose
This study investigated the contribution of white-matter hyperintensities (WMH) and lacunar infarcts (LI) to the risk of Alzheimer's disease (AD) in an elderly cohort in China.
Methods
Older adults who were initially cognitively normal were examined with MRI at baseline, and followed for 5 years. WMH were classified as mild, moderate, or severe, and LI were classified into a few LI (1 to 3) or many LI (≥4). Cognitive function was assessed using the Mini Mental State Examination and the Activities of Daily Living scale.
Results
Among the 2,626 subjects, 357 developed AD by the end of the 5-year follow-up period. After adjusting for age and other potential confounders, having only WMH, having only LI, and having both WMH and LI were associated with an increased risk of developing AD compared with having neither WMH nor LI. Moderate and severe WMH were associated with an increased risk of developing AD compared with no WMH. Furthermore, patients with many LI had an increased risk of developing AD compared with no LI.
Alzheimer's disease (AD) is the most common form of dementia among the elderly and one of the most important health-care challenges in this population. There were reportedly 47 million people worldwide over the age of 60 living with dementia in 2015, and this is expected to increase to 131 million people by 2050.1 There is increasing evidence that many risk factors increase the risk of AD, including being older, low educational attainment, diabetes, hypertension, obesity, smoking, excessive alcohol consumption, and ApoE level.234 It is possible that controlling the related risk factors could greatly reduce the prevalence of dementia, but this remains to be fully explored.5
Cerebral white-matter hyperintensities (WMH) are areas of increased signals in T2-weighted and FLAIR MRI scans. The prevalence of WMH lesions increases with age, and is
associated with arterial hypertension and arteriosclerosis.67 However, studies investigating
the relationship between WMH and dementia have produced inconsistent results. Several
studies have shown that WMH are associated with cognitive decline and dementia in the elderly
population,89 while others have suggested that there are no such relationships.10 Lacunar infarcts (LI) are small (3–20 mm in diameter), noncortical infarcts that are generally thought to be caused by the occlusion of the penetrating arteries.11 However, the association between LI and AD has received little attention. Both WMH and LI are small-vessel lesions, and cranial MRI suggests that the two diseases are highly prevalent in the elderly population. However, the relative impacts of WMH and LI on AD remain unclear.
In the present study we investigated the independent contributions of WMH and LI to the risk of developing AD in an elderly cohort in China. We also examined the associations of the severities of WMH and LI with the risk of developing AD.
The study population included subjects aged at least 60 years who were registered in the Neurology Department of Daping Hospital in Chongqing, China during 2009 and 2010. These subjects underwent brain MRI for the following reasons: transient ischemic attack , stroke, hypertension, diabetes, hyperlipidemia, dizziness, syncope, or headache. Patients with a diagnosis of dementia, a concomitant neurological disorder that could potentially affect cognitive function, a severe and persistent mental illness, or a history of head trauma were excluded from our study. Brain MRI was performed in 3,139 patients. After the initial examination, 261 patients were excluded, resulting in 2,878 patients being followed. The Institutional Review Board of Daping Hospital approved the protocol of this study, and all subjects provided informed consent (IRB No. ChiCTR-OCC-12001966).
The collected demographic data included age, sex, height, weight, and education level, where ≤6 years of schooling was considered to be a low education level and >6 years of schooling was considered to be a high education level. The vascular risk factors included in our analyses were hypertension, hyperlipidemia, diabetes, coronary artery disease, history of stroke, current smoking status, and daily alcohol consumption status. Blood samples were collected to measure the levels of glucose, lipids, high-sensitivity C-reactive protein, homocysteine, and ApoE. Hypertension, diabetes, hyperlipidemia, history of stroke, and coronary artery disease were diagnosed using the tenth revision of the International Classification of Diseases. The smoking status and the alcohol consumption status were classified as described previously.12
The Chinese version of the Mini Mental State Examination (MMSE) and the Activities of Daily Living (ADL) scale were used to measure the cognitive status,13 with cutoff scores of 24 and 16 points, respectively. A battery of neuropsychological examinations was further administered when the MMSE revealed cognitive decline. This battery included the Fuld Object Memory Evaluation to assess extensive cognitive dysfunction,14 Rapid Verbal Retrieval to assess semantic memory,15 the Wechsler Adult Intelligence Scale to assess immediate memory and graphical recognition,16 the Pfeiffer Outpatient Disability Questionnaire to evaluate the ability to engage in social activities,17 the Hamilton Depression Rating Scale to measure emotional status,18 and the Hachinski Ischemic Score (HIS) to detect significant vascular disease.19
A senior neurologist diagnosed AD according to our previously described protocol.13 AD was diagnosed based on the updated clinical diagnostic criteria established by the National Institute on Aging and the Alzheimer's Association.20 The National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria and the HIS were used to distinguish AD from vascular dementia (VaD) as follows: HIS ≤4, AD; 4<HIS<7, mixed dementia; and HIS ≥7, VaD.21 Other dementias such as frontotemporal dementia (FTD) and dementia with Lewy bodies (DLB) were diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders.22
All subjects underwent MRI on a device with a 1.5-T magnet (Signa EXCITE HD, General Electric, Boston, MA, USA) using the following standard protocol: T1-weighted [repetition time (TR)/echo time (TE)=450/8.9 ms], T2-weighted (TR/TE=5,000/87 ms), and FLAIR (TR/TE=8,500/88 ms and inversion time=2,000 ms) scans.
MRI was used to estimate the severity of WMH by rating a FLAIR sequence according to the Fazekas scale, followed by categorization into three severity classes:23 mild (punctate), moderate (early confluent), and severe (confluent). We avoided the confounding presence of focal WMH that were due to index stroke by simultaneously assessing T2-weighted and diffusion-weighted imaging sequences. LI were defined as areas of focal hyperintensity in a T2-weighted sequence that were 3–20 mm in diameter and located in the basal ganglia or subcortical white matter. Areas of WMH were also required to have corresponding prominent hypointensity in a T1-weighted sequence in order to distinguish LI from WMH. The number of LI was classified into a few LI (1 to 3) and many LI (≥4).24 The presence of both WMH and LI was defined when WMH and LI existed together.
Automatic quantification of intracranial volume, brain parenchyma volume, and WMH volume was performed using T2-weighted and FLAIR images with Software for Neuro-Image Processing in Experimental Research.25 A percentage measure reflecting the acquired loss of brain volume was calculated using the following equation:
In total, 2,878 patients were followed for 5 years from January 2011 to December 2015. The same neuropsychological tests were performed annually by a neuropsychologist blinded to all clinical information.
The chi-square test and Fisher's exact test were used to analyze categorical data. Continuous data were analyzed with t tests or one-way ANOVA followed by the Tukey's tau-b for multiple comparisons. Between-groups analyses of baseline variables were performed with a chi-square test, Fisher's exact test, t test, and Mann-Whitney U test. The Cox proportional-hazards model was used to estimate hazard ratios (HRs) for AD. The models were first applied without adjusting for any relevant confounding factors. They were subsequently adjusted for the covariates that were chosen a priori, including age, sex, education level, hypertension, hyperlipidemia, diabetes, smoking status, alcohol consumption status, and ApoE level. All analyses were conducted using SPSS 19.0 for Windows (IBM Corp., Armonk, NY, USA).
During the 5-year follow-up period, 89 of the patients died, 67 moved away, and 96 discontinued participation (57 with VaD, 13 with DLB, 7 with FTD, and the remaining 19 patients dropped out due to the presence of severe illnesses such as cardiac, hepatic, renal failure, or other systemic diseases), leaving 2,626 patients who completed the 5-year follow-up. AD was diagnosed in 357 (13.6%) of these patients. MRI was performed at the end of the 5-year follow-up in 2009 (76.5%) patients, of which 260 (72.8%) patients were in the AD group, and 186 (71.5%) patients were found to have brain atrophy.
The age of the patients was 66.3±8.1 years (mean±SD) at baseline, and 51.6% of them were female. Among the 2,626 patients, only WMH were found in 856 (32.6%) patients, only LI were found in 496 (18.9%) patients, and both WMH and LI were found in 407 (15.5%) patients. The baseline characteristics of the study participants are summarized in Table 1. The patients with AD were older, more likely to be female and have a low education level, hypertension, hyperlipidemia, and diabetes, be current smokers and daily drinkers, and have higher plasma ApoE levels.
The severities of WMH and LI are compared between participants with and without AD in Table 2. At the end of the follow-up, 147 patients with WMH had developed AD, including 59 (36.6%) with mild WMH, 50 (37.4%) with moderate WMH, and 38 (26.0%) with severe WMH. Additionally, 80 patients with LI developed AD, including 46 (43.0%) with a few LI and 37 (34.6%) with many LI. The severities of WMH and LI differed significantly between the patients who did and did not develop AD. Furthermore, significantly higher proportions of AD patients had only WMH, only LI, or both WMH and LI than neither WMH nor LI. The number of patients with WMH and LI had also increased at the end of the 5-year follow-up. Among 357 AD patients, there had been 147 patients with only WMH at baseline, and this increased to 163 at the end of the follow-up; similarly, the number with only LI increased from 80 to 87, and the number with both WMH and LI increased from 81 to 85.
The associations of AD with WMH and LI are presented in Table 3 for the Cox proportional-hazards regression model. After adjusting for age, sex, education level, hypertension, hyperlipidemia, diabetes, smoking status, alcohol consumption status, and ApoE level, moderate WMH (HR=1.31, 95% CI=1.27–1.52) and severe WMH (HR= 1.75, 95% CI=1.37–2.01) were associated with an increased risk of developing AD when compared with no WMH. The presence of many LI (HR=1.34, 95% CI=1.26–1.52) was also associated with an increased risk of developing AD when compared with no LI after adjusting for confounding factors. Furthermore, the presence of only WMH (HR=1.40, 95% CI=1.25–1.59), the presence of only LI (HR=1.23, 95% CI=1.14–1.39), and the presence of both WMH and LI (HR=2.03, 95% CI=1.69–2.26) were found to be associated with an increased risk of developing AD when compared with the presence of neither WMH nor LI after adjusting for confounding factors.
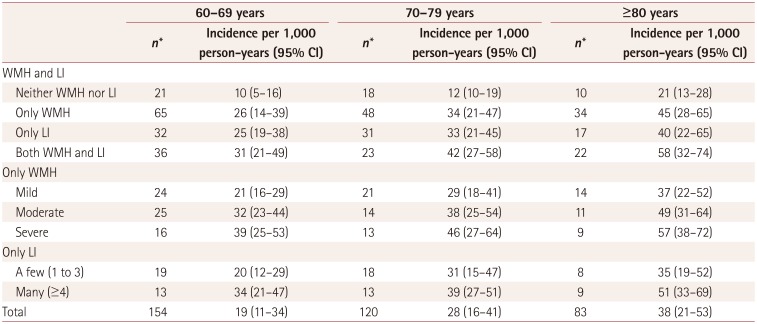
The age-stratified relationships of the presence of only WMH, only LI, and both WMH and LI with the incidence of AD are presented in Table 4. All patients were divided into three age groups: 60–69, 70–79, and ≥80 years. A particularly interesting finding was that in each age group, the incidence of AD per 1,000 person-years was significantly higher in patients with only WMH, only LI, and both WMH and LI than in patients with neither WMH nor LI (p<0.01). Furthermore, in each age group the incidence of AD per 1,000 person-years was significantly higher in patients with moderate or severe WMH than in patients with mild WMH (p<0.01). Finally, the incidence of AD per 1,000 person-years was also significantly higher in patients with many LI than in patients with a few LI (p<0.05).
Fig. 1 illustrates the incidence rates of AD in patients with only WMH, only LI, or both WMH and LI during the 5-year follow-up period. At the end of the follow-up period, the incidence of AD was significantly higher in patients with only WMH, only LI, or both WMH and LI than in those with neither WMH nor LI (p<0.01).
Fig. 2 shows the MMSE and ADL scores in patients with only WMH, only LI, or both WMH and LI at the end of the follow-up. Patients with only WMH, only LI, or both WMH and LI had significantly lower MMSE scores than those with neither WMH nor LI (p<0.01) (Fig. 2A). In addition, patients with only WMH, only LI, or both WMH and LI had significantly higher ADL scores than those with neither WMH nor LI (p<0.01) (Fig. 2B).
Previous studies have found an association between WMH and AD.27 The Cardiovascular Health Cognition Study investigated the relationship between WMH and the risk of dementia in 3,608 participants who underwent MRI examinations of the skull over an 8-year follow-up period.28 That study found that participants with a higher severity of WMH had an increased risk of developing clinical dementia (HR=2.0, 95% CI=1.63–2.37). The Rotterdam Scan Study also found WMH to be strongly associated with an increased risk of developing dementia.29 Another longitudinal study of 85 patients with uncomplicated AD conducted over a 6-month follow-up period found that WMH were associated with a greater degree of cognitive impairment in patients with AD.30 However, another study found that there was no significant association between WMH and the risk of clinical dementia in 204 participants who were followed as part of the Osaki-Tajiri Project over a 5-year period.10 The present prospective and large-sample study found that moderate and severe WMH were associated with an increased risk of developing AD.
Few studies have directly investigated the relationship between LI and the risk of developing dementia.3132 Kitagawa et al.31 retrospectively examined the impact of LI on the incidence of dementia in 1,106 patients with vascular risk factors, and found a significant association between LI and dementia after adjusting for relevant confounding factors. Vermeer et al.32 prospectively examined the association between LI and the risk of dementia in 1,015 participants aged 60–90 years and free of dementia at baseline, and found LI to be strongly associated with an increased risk of the development of dementia over time. Our results also suggest that patients with many LI have an increased risk of AD.
WMH and LI are considered to be cerebral small-vessel diseases,11 and they are often present in patients with vascular risk factors.3334 There is increasing evidence that vascular risk factors play a role in the progression of AD.212 Qiao et al.35 explored the correlation between vascular risk factors and AD in 123 Chinese outpatients with probable AD who were followed for 3 years, and found hypertension, diabetes, hyperlipidemia, stroke, and coronary artery disease to be common comorbidities in patients with AD. In the present study, both WMH and LI were associated with a higher risk of developing AD compared with only WMH and only LI, suggesting that WMH and LI exert certain synergistic effects on the development of AD. These effects might be due to WMH being associated with global neurocognitive deficits and psychomotor speed,36 whereas LI individually contribute to a significant deterioration of domain-specific impairments such as mental and motor speed, and executive functions.37 Therefore, patients with both WMH and LI simultaneously will have even greater cognitive impairment, which markedly increases the risk of clinical dementia.
Cerebral WMH involve axonal loss and demyelination, and their pathogenesis is thought to be due to ischemia related to small-vessel diseases. However, WMH may also result from the consequences of cortical AD pathology [i.e., beta-amyloid (Aβ) deposition and hyperphosphorylated tau].383940 To elucidate the association between WMH and the progression of Aβ deposition in patients with AD, Grimmer et al.39 performed FLAIR MRI and [11C]PiB PET after a 28-month follow-up of 22 patients with probable AD at baseline. Their longitudinal analysis revealed a statistically significant association between WMH and the progression of the Aβ load (p=0.006). Another study that used postmortem MRI showed that an increase in the cortical hyperphosphorylated tau burden independently predicted the severity of WMH in the pathogenesis of WMH damage.40 The findings of that study also suggested that the presence of WMH is a marker for cortical AD-associated pathology rather than small-vessel disease in clinical dementia patients.
Several studies have also demonstrated an association between LI and AD.4142 One study found an association between small-vessel disease and AD pathology, which was further associated with cognitive decline and dementia over 13-year time period (from January 2002 to December 2012).41 Another study examined the associations of WMH and LI with the CSF levels of Aβ42 and phosphorylated tau in 914 consecutive patients for which CSF and MRI scans were available, with the results showing that the deposition of amyloid was aggravated in patients with cerebral small-vessel disease. Another cross-sectional study demonstrated that the presence of LI was tightly coupled to an increase in the plasma Aβ40 concentration in patients with AD.42
The strengths of the present study are that it included a large number of elderly people from the general population, its prospective design, and to it being (to our knowledge) the first study to investigate the associations of WMH, LI, and their synergistic effects with an increased risk of developing AD. However, this study was subject to some limitations. First, we did not use magnetic resonance diffusion-tensor imaging (DTI) to detect damage to the white matter. DTI is a new method for revealing the structure of white matter in the brain that can detect subtle WMH in white-matter tracts.43 Using DTI to observe changes in these tracts might have demonstrated a more-robust relationship between WMH and the risk of developing AD. Second, previous studies have suggested that periventricular WMH play an independent role in the development of dementia, especially with regards to AD,44 and we did not divide WMH into periventricular WMH and deep WMH in our analyses. Third, the severity of LI as well as their distribution (i.e., whether they are found in the subcortex, cerebral cortex, brainstem, or cerebellum) have been shown to be related to the risk of cognitive decline.4546 However, we did not examine the relationship between the distribution of LI and the risk of developing AD. Fourth, previous studies have suggested that WMH and LI exert different effects on cognitive function. Although this study evaluated various cognitive functions, such as semantic memory and transient memory, the associations of WMH and LI with various cognitive impairments were not assessed. Establishing relationships of LI and WMH with cognitive decline in specific cognitive areas would be highly significant to revealing the correlations of LI and WMH with the increased risk of developing AD. Fifth, this observational study only investigated the associations of WMH, LI, and their synergistic effects with the development of AD, and did not further explore the impact of medication on the development of AD. Finally, changes in the Aβ level in CSF have been shown to be associated with risk of developing AD,47 and some studies have revealed that such level changes are also associated with WMH.48 However, we did not detect changes in the Aβ level in CSF.
In conclusion, this large-sample, prospective clinical study has demonstrated that WMH and LI are associated with the risk of developing AD. The results suggest that controlling the progression of brain white-matter lesions and cavities may reduce the risk of developing AD. The mechanisms underlying how WMH and LI lead to the development of AD warrant further investigations.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81571258).
The authors would like to thank and acknowledge all of the participants who were enrolled in the study.
References
1. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015; 11:718–726. PMID: 26045020.

2. Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, et al. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS One. 2014; 9:e87095. PMID: 24489845.

3. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012; 2012:367516. PMID: 23243502.

4. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014; 13:788–794. PMID: 25030513.

5. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011; 377:1019–1031. PMID: 21371747.

6. Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, et al. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci. 2015; 7:221. PMID: 26648866.

7. Park JH, Ovbiagele B. Response to the comment ‘conflicting evidence on the association of white matter hyperintensities with large-artery disease’. Eur J Neurol. 2015; 22:e81. PMID: 26371442.

8. Nolze-Charron G, Mouiha A, Duchesne S, Bocti C. Alzheimer's Disease Neuroimaging Initiative. White matter hyperintensities in mild cognitive impairment and lower risk of cognitive decline. J Alzheimers Dis. 2015; 46:855–862. PMID: 26402625.

9. Altamura C, Scrascia F, Quattrocchi CC, Errante Y, Gangemi E, Curcio G, et al. Regional MRI diffusion, white-matter hyperintensities, and cognitive function in Alzheimer's disease and vascular dementia. J Clin Neurol. 2016; 12:201–208. PMID: 27074295.

10. Meguro K, Ishii H, Kasuya M, Akanuma K, Meguro M, Kasai M, et al. Incidence of dementia and associated risk factors in Japan: The Osaki-Tajiri Project. J Neurol Sci. 2007; 260:175–182. PMID: 17553526.

11. Zhang X, Ding L, Yuan J, Qin W, Hu W. Spatial relationship between acute lacunar infarction and white matter hyperintensities. Eur Neurol. 2015; 74:259–266. PMID: 26645081.

12. Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H. Association of smoking and alcohol drinking with dementia risk among elderly men in China. Curr Alzheimer Res. 2014; 11:899–907. PMID: 25274108.

13. Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004; 251:421–427. PMID: 15083286.

14. Fuld PA. The fuld object-memory evaluation. Chicago: Stoelting Instrument Co.;1981.
15. Zhang M, Qu G, Wang Z, Cai G, Katzman R, Simon D, et al. Prevalence study on dementia and Alzheimer disease. Zhonghua Yi Xue Za Zhi. 1990; 70:424–428. PMID: 2174278.
16. Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1992; 49:448–452. PMID: 1580805.
17. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975; 23:433–441. PMID: 1159263.

18. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23:56–62. PMID: 14399272.

19. Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006; 37:2220–2241. PMID: 16917086.

20. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269. PMID: 21514250.

21. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993; 43:250–260. PMID: 8094895.

22. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington: American Psychiatric Association;2000.
23. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356. PMID: 3496763.

24. van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005; 36:2116–2120. PMID: 16141425.
25. Admiraal-Behloul F, van den Heuvel DM, Olofsen H, van Osch MJ, van der Grond J, van Buchem MA, et al. Fully automatic segmentation of white matter hyperintensities in MR images of the elderly. Neuroimage. 2005; 28:607–617. PMID: 16129626.

26. van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, Bollen EL, Westendorp RG, van Buchem MA, et al. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann Neurol. 2002; 52:62–67. PMID: 12112048.

27. Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging. 2015; 36:27–32. PMID: 25155654.

28. Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003; 22:13–22. PMID: 12566949.

29. Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004; 61:1531–1534. PMID: 15477506.

30. Diaz JF, Merskey H, Hachinski VC, Lee DH, Boniferro M, Wong CJ, et al. Improved recognition of leukoaraiosis and cognitive impairment in Alzheimer's disease. Arch Neurol. 1991; 48:1022–1025. PMID: 1929892.

31. Kitagawa K, Miwa K, Yagita Y, Okazaki S, Sakaguchi M, Mochizuki H. Association between carotid stenosis or lacunar infarction and incident dementia in patients with vascular risk factors. Eur J Neurol. 2015; 22:187–192. PMID: 25164480.

32. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003; 348:1215–1222. PMID: 12660385.

33. Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014; 82:1331–1338. PMID: 24623838.

34. Rutten-Jacobs LC, Markus HS. UK Young Lacunar Stroke DNA Study. Vascular risk factor profiles differ between magnetic resonance imaging-defined subtypes of younger-onset lacunar stroke. Stroke. 2017; 48:2405–2411. PMID: 28765289.

35. Qiao J, Lu WH, Wang J, Guo XJ, Qu QM. Vascular risk factors aggravate the progression of Alzheimer's disease: a 3-year follow-up study of Chinese population. Am J Alzheimers Dis Other Demen. 2014; 29:521–525. PMID: 24562899.
36. Xu X, Hilal S, Collinson SL, Chong EJ, Ikram MK, Venketasubramanian N, et al. Association of magnetic resonance imaging markers of cerebrovascular disease burden and cognition. Stroke. 2015; 46:2808–2814. PMID: 26330446.

37. Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 2011; 76:1872–1878. PMID: 21543730.

38. McAleese KE, Walker L, Graham S, Moya EL, Johnson M, Erskine D, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017; 134:459–473. PMID: 28638989.

39. Grimmer T, Faust M, Auer F, Alexopoulos P, Förstl H, Henriksen G, et al. White matter hyperintensities predict amyloid increase in Alzheimer’s disease. Neurobiol Aging. 2012; 33:2766–2773. PMID: 22410648.

40. McAleese KE, Firbank M, Dey M, Colloby SJ, Walker L, Johnson M, et al. Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol Commun. 2015; 3:60. PMID: 26419828.

41. Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 2014; 71:855–862. PMID: 24818585.

42. Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006; 66:23–29. PMID: 16401840.
43. Pelletier A, Periot O, Dilharreguy B, Hiba B, Bordessoules M, Chanraud S, et al. Age-related modifications of diffusion tensor imaging parameters and white matter hyperintensities as inter-dependent processes. Front Aging Neurosci. 2016; 7:255. PMID: 26834625.

44. Kim S, Choi SH, Lee YM, Kim MJ, Kim YD, Kim JY, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr. 2015; 27:2069–2077. PMID: 26212042.

45. Chen Y, Wang J, Zhang J, Zhang T, Chen K, Fleisher A, et al. Aberrant functional networks connectivity and structural atrophy in silent lacunar infarcts: relationship with cognitive impairments. J Alzheimers Dis. 2014; 42:841–850. PMID: 24946874.

46. Nho K, Saykin AJ, Nelson PT. Alzheimer's Disease Neuroimaging Initiative. Hippocampal sclerosis of aging, a common Alzheimer's disease ‘mimic’: risk genotypes are associated with brain atrophy outside the temporal lobe. J Alzheimers Dis. 2016; 52:373–383. PMID: 27003218.

47. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356. PMID: 12130773.

48. Roseborough A, Ramirez J, Black SE, Edwards JD. Associations between amyloid β and white matter hyperintensities: a systematic review. Alzheimers Dement. 2017; 13:1154–1167. PMID: 28322203.

Fig. 1
Incidence of AD during the 5-year follow-up. At the end of the follow-up, the incidence of AD dementia was significantly higher in patients with only WMH, only LI, and both WMH and LI than in those with neither WMH nor LI (p<0.01). AD: Alzheimer's disease, LI: lacunar infarcts, WMH: white-matter hyperintensities.
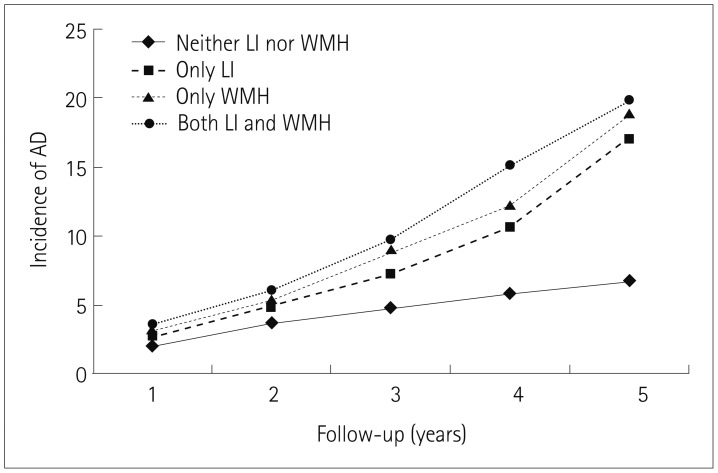
Fig. 2
Relationships of WMH and LI with cognitive function scores at the end of the follow-up. A: Patients with only WMH, only LI, and both WMH and LI had lower mean MMSE scores than those with neither WMH nor LI (p<0.01). B: Patients with only WMH, only LI, and both WMH and LI had higher mean ADL scores than those with neither WMH nor LI (p<0.01). ADL: Activities of Daily Living, LI: lacunar infarcts, MMSE: Mini Mental State Examination, WMH: white-matter hyperintensities.

Table 1
Comparison of baseline characteristics between patients with and without AD
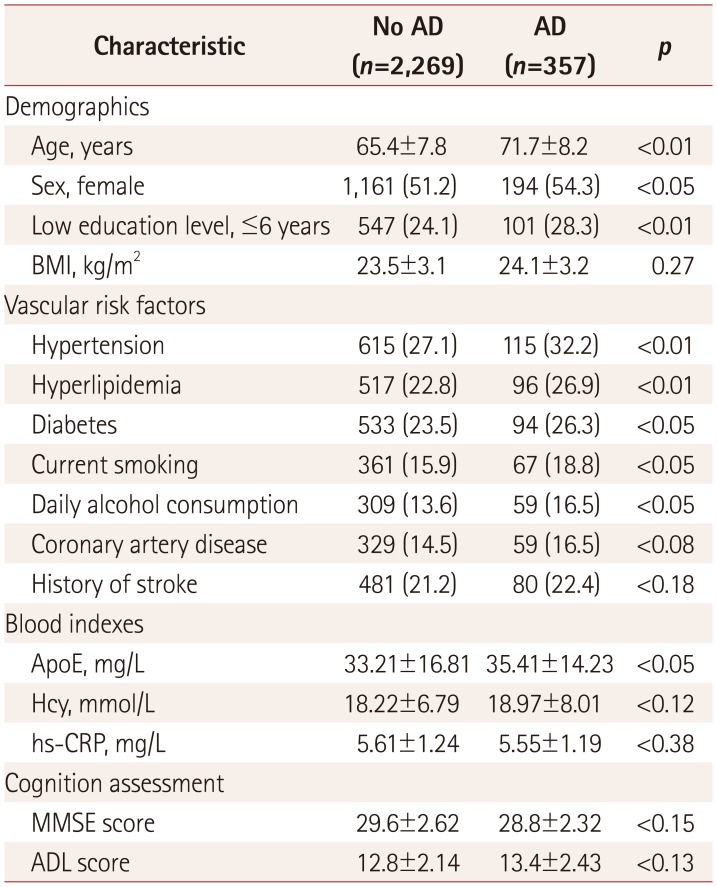
Table 2
Comparison of severities of WMH and LI between patients with and without AD
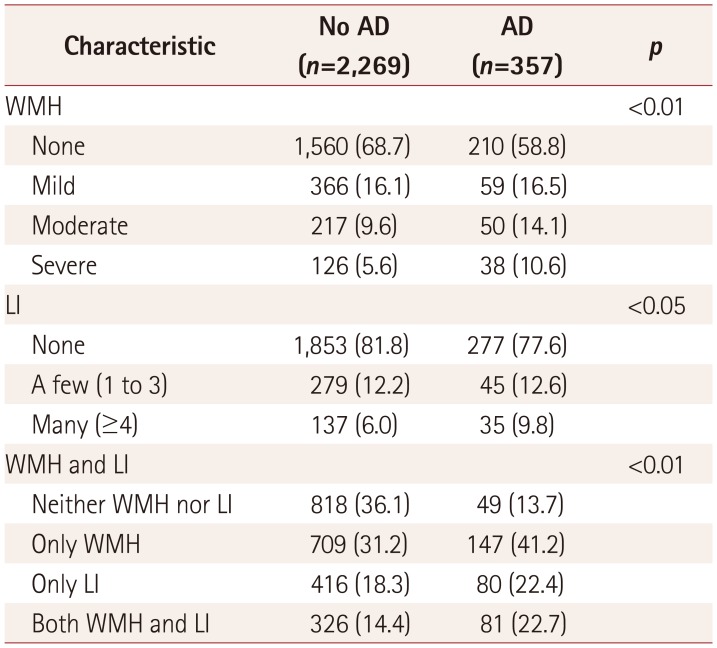
Table 3
Associations of WMH and LI with AD in a Cox proportional-hazards regression model
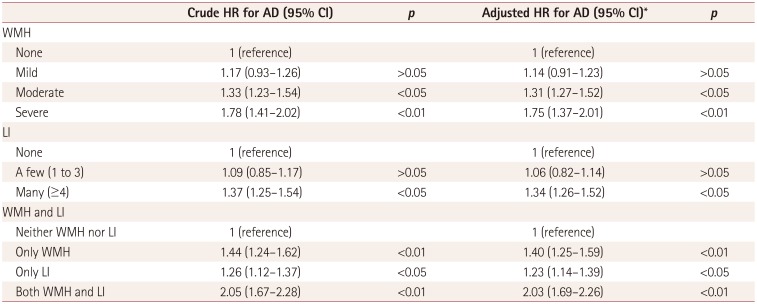
Table 4
Relationships of WMH and LI with the incidence of AD according to age




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download