Abstract
Background
This study examined the outcomes of ABO incompatible living donor liver transplantation (LDLT). The changes in the immunologic factors that might help predict the long term outcomes were also studied.
Methods
Twenty-three patients, who underwent ABO incompatible LDLT from 2010 to 2015, were reviewed retrospectively. The protocol was the same as for ABO compatible LDLT except for the administration of rituximab and plasma exchange. The clinical outcomes and immunologic factors, such as isoagglutinin titer and cluster of differentiation 20+ (CD20+) lymphocyte levels were reviewed.
Results
The center showed a 3-year survival of 64% with no case of antibody-mediated rejection. When transplantation-unrelated mortalities (for example, traffic accidents and myocardial infarction) were removed from statistical analysis, the 3-year survival was 77.8%. Although isoagglutinin titers continued to remain at low levels, the CD20+ lymphocyte levels recovered to the pre-Rituximab levels at postoperative one year.
Conclusions
As donor shortages continue, ABO incompatible liver transplantation is a feasible method to expand the donor pool. On the other hand, caution is still needed until more long-term outcomes are reported. Because CD20+ lymphocytes are recovered with time, more immunologic studies will be needed in the future.
Liver transplantation (LT) is the treatment of choice for end-stage liver disease and selected patients with hepatocellular carcinoma (HCC). However, due to the problem of organ shortage, efforts have been made to expand the donor pool, leading to living donor liver transplantation (LDLT). With continuous problem of organ shortage, ABO incompatible LDLT, once considered a contraindication, is being performed. Starzl et al.(1) have reported 11 cases of ABO incompatible LT in 1979 and suggested that the liver is an immune privileged organ. However, reports of poor outcomes soon followed, showing low rates of graft survival and high incidence of primary humoral rejection with hemorrhagic infiltration of portal tracts(23). With lessons learned from advances in ABO incompatible kidney transplantation, over immunosuppression, splenectomy, graft local injections, and plasma exchange have been used to improve outcomes of ABO incompatible LT. In addition to these strategies, the use of rituximab to reduce B cells was started in 2003. It is now widely used to overcome antibody mediated rejection(4). Even with these advances, ABO incompatible LT is reserved for emergency cases(5).
Recently, ABO incompatible LDLT is being performed at many centers. Many reports have shown that results of ABO incompatible LT are comparable to those of ABO compatible LT(6). However, these reports are about short-term outcomes. Long-term outcomes are lacking. Studies on immunologic factors such as isoagglutinin titer and cluster of differentiation 20+ (CD20+) lymphocytes that may help predict long term outcomes are also lacking. Therefore, the objective of this study was to evaluate changes of isoagglutinin titer and CD20+ lymphocytes and results of ABO incompatible LDLT at our institution.
Between October 2010 and September 2015, 272 LDLT were performed at our center. Of these patients, 23 (8.5%) received ABO incompatible LDLT. These 23 patients were retrospectively reviewed. Patients' demographics, model for end-stage liver disease (MELD) score, donor and recipient ABO types, survival time, complications, cause of mortality, isoagglutinin titers, CD20+ lymphocyte levels, and plasma exchange numbers were reviewed. Median follow up period was 22 months (range, 0~71). Patients were not required to give informed consent to the study because the analysis used anonymous clinical datat that were obtained after each paitent agreed to treatment by written consent. This study was approved by the Institutional Review Board of Catholic Medical Center (KC17RCSI0506).
Our institution's protocol begins with a single injection of rituximab (300 mg/m2) 2 weeks prior to transplantation. There were two cases of emergency ABO incompatible LDLT in which rituximab was administered just 1 week before the operation. Repeated sessions of plasma exchange with a target titer of 1:16 were then performed. The protocol for ABO incompatible LDLT is the same as that for ABO compatible LDLT except for pre-LT desensitization (rituximab, plasma exchange). Immunosuppression regimen included tacrolimus, mycophenolate mofetil (MMF), and steroids. The dose of tacrolimus was adjusted to maintain level at 7 to 10 ng/mL for the first postoperative month and 5 to 7 ng/mL thereafter. Steroids were withdrawn at 1 month after surgery and MMF was withdrawn at 6 months after transplantation. Basiliximab was administered on operation day and postoperative day 4. Prostaglandin E1 and gabexate were administered at the same dose as for ABO compatible LT. Intravenous immunoglobulin was used for early cases. However, it is now no longer used. Splenectomy or local graft infusion was never part of our protocol.
Patients with hepatitis B virus (HBV) were treated with hepatitis B immunoglobulin (HBIG) and nucleos(t)ide analogues. Patients were given 10,000 units of HBIG during the anhepatic phase, which was followed daily for 7 days and then every month for 6 months after transplantation. Afterwards, the patients were given 4,000 units of HBIG intravenously every month. A target hepatitis B surface antibody (HBsAb) level was 200 IU/L in our protocol. Nucleos (t)ide analogues were started 3 days after transplantation.
Transplantation was performed according to standard technique using right lobe with middle hepatic vein reconstruction. Detailed methods have been described previously(78). The surgical procedure itself had no difference from that for ABO compatible LT. All patients were treated with a standardized postoperative protocol. Doppler ultrasound was performed every other day during the first week and weekly thereafter during the first month. On the 7th and 20th postoperative days, follow-up liver computed tomography (CT) scans were performed to evaluate vascular status and liver regeneration. On the 20th postoperative day, magnetic resonance cholangiopancreatography (MRCP) was performed to evaluate biliary status. CT scans were performed annually thereafter or in the presence of abnormal clinical conditions. MRCP was performed when biliary complications were suspected. Biliary stricture was diagnosed when stenosis of the bile duct and dilatation of the intrahepatic duct proximal to the stricture were observed on abdominal CT or MRCP and when liver biochemical parameters such as serum bilirubin, alkaline phosphatase, and γ-glutamyl transferase were abnormal. In HCC patients, tumor markers were measured monthly during the first year, and then every 2 months thereafter. Abdomen CT, chest CT, and bone scintigraphy were routinely performed every 4 months for the first year, every 6 months for the second year, and then annually thereafter. When tumor recurrence was suspected, magnetic resonance imaging and/or positron emission tomography-CT were performed. Real-time quantitative polymerase chain reaction (PCR) for cytomegalovirus (CMV) was checked twice per week until discharge. CMV infection was diagnosed if PCR showed positive results, regardless of symptoms. When CMV infection was diagnosed, empirical ganciclovir was administered until CMV was no longer detected on PCR. Patient's antibody titer was checked every day for the first 2 weeks, every other day for the next 2 weeks, and every month thereafter. CD20+ lymphocyte levels were checked during preparation for transplantation, at time of transplantation, and every month after transplantation. CD20 lymphocyte level was tested with patients' blood samples. Flow cytometry was used and ratio of CD20 lymphocytes to total lymphocytes is shown as percentage.
Continuous variables are reported as mean±standard deviation. Non-normal distribution variables are reported as medians. Overall survival was calculated using the Kaplan–Meier method. Statistical analyses were performed using SPSS software ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was considered at P<0.05.
A total of 23 patients with a mean age of 53.1±9.3 years were enrolled, including 17 males (73.9%) (Table 1). Hepatitis B was the most common cause of transplantation, accounting for 17 cases (73.9%). Fourteen patients (60.9%) had HCC, including eight patients (57.1%) who had transplantation beyond Milan criteria. Two patients diagnosed with HCC before transplantation turned out to be cholangiocarcinoma. Mean MELD score was 10.9±6.3 (median, 10; range, 7 to 26). The most common ABO type was B donor to O recipient, having seven cases. Median values of initial immunoglobulin M (IgM) and immunoglobulin G (IgG) anti-ABO isoagglutinin titers were 1:64 (range, 1:2 to 1:512) and 1:128 (range, 1:16 to 1:2,048), respectively. Median follow-up period was 22 months.
Median values of initial IgM and IgG isoagglutinin titers were 1:64 (range, 1:2 to 1:512) and 1:128 (range, 1:16 to 1:2,048), respectively (Fig. 1). After several sessions of plasma exchange, median IgM and IgG titers at time of transplantation were 1:4 (range, negative to 1:64) and 1:16 (range, 1:2 to 1:256), respectively. The median number of plasma exchange sessions was 4 (range, 2 to 17). Anti-ABO isoagglutinin titer decreased even more after transplantation, showing median values of 1:2 (range, negative to 1:32) for IgM and 1:8 (range, 1:1 to 1:64) for IgG. The titer did not increase during the follow-up period. At postoperative one year, median values were 1:2 (range, 1:1 to 1:16) for IgM and 1:8 (range, 1:1 to 1:32) for IgG.
The median proportion of CD20+ lymphocytes in peripheral blood was 15%. It was reduced to undetectable levels after administration of rituximab (Fig. 2). During follow-up, CD20+ lymphocyte levels were generally lower than those before administration of rituximab. However, CD20+ lymphocyte level showed an increase at 3 months (median 4%) and nearly recovered to pre-rituximab levels at 1-year follow-up.
This study showed 1-year survival of 73%, 2-year survival of 64%, and 3-year survival of 64% (Fig. 3). A total of nine mortalities occurred. The cause of death was sepsis in two patients, cholangiocarcinoma recurrence in two patients, graft failure in one patient, graft rejection caused by noncompliance in one patient, graft-versus-host disease (GVHD) in one patient, myocardial infarction in one patient, and traffic accident in one patient (Table 2).
No antibody mediated rejection was observed, even in two cases of emergency transplantation in which rituximab was administered just 1 week prior to operation. There were eight cases of biliary stricture. However, diffuse intrahepatic biliary stricture sometimes reported in ABO incompatible LT was not observed in any case. Two cases of CMV infection and one case of post-operative bleeding occurred.
Early reports of ABO incompatible LDLT showed unfavorable outcomes and ABO incompatible transplantation was considered a contraindication(23). Hyper-acute antibody mediated rejection presenting as single organ disseminated intravascular coagulation was reported as the main barrier to ABO incompatible LT(9). Better understanding of immunity and the use of rituximab, graft local infusion, splenectomy, and plasma exchange have decreased antibody mediated rejection(41011). Recent reports have shown that results of ABO incompatible LT are comparable to those of ABO compatible LT(61112131415).
At most centers, the backbone of ABO incompatible transplantation protocol is rituximab with plasma exchange. However, there is no consensus on the timing or dose of rituximab administration. Our center administered 300 mg/m2 at 2 weeks before operation except for two cases of emergency transplantation in which the patients were administered with rituximab one week prior to operation. Other centers also used 300 mg/m2 of rituximab at 2 weeks before operation(14), although dose of 375 mg/m2 at 2 weeks before operation(15), dose of 300 mg/m2 at least 7 days before operation(6), and dose of 375 mg/m2 at 10 days before operation(13) have also been reported. Song et al.(12) have reported that dose of 375 mg/m2 at 2 to 3 weeks before operation was used for early cases. Later it was reduced to 300 mg/m2. There is no consensus on isoagglutinin titer or plasma exchange either. Target isoagglutinin titer before transplantation was 1:16 at our center, although other studies have reported a target of 1:8(12) to 1:32(6). In one study, Kim et al.(15) have postponed the operation if the target titer of 1:8 is not met. At our center, one patient (case 13) showed IgM titer of 1:4 and IgG titer of 1:256 after 12 sessions of plasma exchange and transplantation was performed (Table 3). There was no complication and the patient was discharged well. Some centers have even reported that ABO incompatible LT can be safely performed with rituximab alone without plasma exchange(16). Splenectomy and local graft infusion are part of the protocol at some centers. However, there are complications. After reports showing acceptable results without splenectomy or local graft infusion(1718), These two methods are largely abandoned now. Our center did not perform splenectomy or local graft infusion from the beginning.
During follow-up period, nine deaths occurred. Our study showed 1-year survival of 73.9%, 2-year survival of 64.7%, 3-year survival of 64.7%, and 5-year survival of 56.6%. These survival rates are lower than those of recent reports in our country. One study(12) reported 3-year survival of 92.3% while another study(15) had 10-month survival of 100%. The reason for this might be because we enrolled higher proportion of advanced HCC patients compared to other studies. Moreover, in our study, some mortalities were due to unexpected causes not directly related to transplantation. Two deaths were due to sepsis. There were two cases of death caused by cholangiocarcinoma recurrence. One patient, a 58-year-old male (case 6), was diagnosed with HCC(B) and treated with transarterial chemoembolization (TACE) three times. At first, TACE seemed effective and the lesion regressed. Eventually, LT was performed due to progression on follow-up imaging studies. After the operation, the lesion was HCC with combined cholangiocarcinoma. Another patient was a 54-year-old male treated with TACE nine times. He eventually underwent ABO incompatible LT. The pathology turned out to be cholangiocarcinoma. One mortality (case 7) was due to graft failure. The patient died even after re-transplantation with cadaveric graft. Another patient (case 16) had major depressive disorder. At 8 months after LT, the patient was lost to follow-up and admitted via emergency room 5 months later for liver failure and expired. One patient (case 17) was admitted for diarrhea and skin rash, and treated for suspicious pseudomembranous colitis and drug eruption. The patient later developed pancytopenia and died from eventual pneumonia. After careful review of the patient, we concluded that the patient suffered GVHD. In addition to the symptoms of diarrhea, rash, and pancytopenia, the donor's human leukocyte antigen (HLA) type was monozygotic haplotype of the recipient, which is a risk factor for GVHD. One patient (case 8) had patent liver function 3 years after transplantation but died suddenly from myocardial infarction. One patient was well at 1 year after transplantation. However, the patient died from a traffic accident. A more careful patient selection might improve outcomes of ABO incompatible LT. After removing cases of unexpected deaths due to transplantation-unrelated causes in statistical analysis, our center showed 3-year overall survival after ABO incompatible LT was 77.8%.
This study has the shortcomings of being a retrospective study with small number of patients and a short follow-up period. However, we studied changes of immunologic factors over time. Such studies are insufficient in the literature. Anti-ABO isoagglutinin titers and CD20+ lymphocyte levels are related to antibody mediated rejection. They might be useful for predicting long term outcomes. In our study, isoagglutinin titers remained suppressed at 1 year regardless of donor/recipient ABO types. In other studies in the literature that checked titers after transplantation, four of 20 patients(14) and two of 15 patients(10) showed temporary increase in isoagglutinin titers after transplantation. Unlike isoagglutinin titers, CD20+ lymphocytes levels began to increase at postoperative 3 months and recovered to almost initial levels at 1-year. Above mentioned studies(1014) showed rebound increase of CD19+ B cells at around 6 and 8 months. Since B cells play an important role in antibody mediated rejection, this result calls for caution. More long-term studies are needed.
Figures and Tables
Fig. 1
Change of isoagglutinin titer in ABO incompatible living donor liver transplantation. (A) Immunoglobulin M (IgM) isoagglutinin titer, (B) immunoglobulin G (IgG) isoagglutinin titer. Abbreviations: Neg, negative; OP, operation.

Fig. 2
Change of CD20+ lymphocytes in ABO incompatible living donor liver transplantation. Abbreviation: OP, operation.
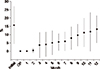
Table 3
Clinical outcomesof patients who underwent ABO incompatible living donor liver transplantation

Abbreviations: HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; IgM, immunoglobulin M; IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; F/U, follow-up; CC, cholangiocarcinoma; CMV, cytomegalovirus; Neg, negative; AHF, acute hepatic failure; GVHD, graft-versus-host disease.
aB: beyond the Milan criteria, W: within the Milan criteria; bStricture: biliary stricture.
References
1. Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GP, Porter KA, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979; 77:375–388.

2. Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990; 336:519–523.

3. Rego J, Prevost F, Rumeau JL, Modesto A, Fourtanier G, Durand D, et al. Hyperacute rejection after ABO-incompatible orthotopic liver transplantation. Transplant Proc. 1987; 19:4589–4590.
4. Monteiro I, McLoughlin LM, Fisher A, de la Torre AN, Koneru B. Rituximab with plasmapheresis and splenectomy in abo-incompatible liver transplantation. Transplantation. 2003; 76:1648–1649.

5. Stewart ZA, Locke JE, Montgomery RA, Singer AL, Cameron AM, Segev DL. ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver Transpl. 2009; 15:883–893.

6. Kim JD, Choi DL, Kim SG, Lee AJ. Single-center experience of ABO-incompatible living-donor liver transplantation with a new simplified intravenous immunoglobulin protocol: a propensity score-matching analysis. Transplant Proc. 2016; 48:1134–1138.

7. Kim SJ, Na GH, Choi HJ, Yoo YK, Kim DG. Surgical outcome of right liver donors in living donor liver transplantation: single-center experience with 500 cases. J Gastrointest Surg. 2012; 16:1160–1170.

8. Na GH, Kim DG, Choi HJ, Han JH, Hong TH, You YK. Interventional treatment of a biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis. HPB (Oxford). 2014; 16:312–319.

9. Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988; 132:489–502.
10. Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, et al. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011; 92:1134–1139.

11. Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005; 79:12–16.

12. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016; 16:157–170.

13. Lee J, Lee JG, Lee JJ, Kim MS, Ju MK, Choi GH, et al. Results of ABO-incompatible liver transplantation using a simplified protocol at a single institution. Transplant Proc. 2015; 47:723–726.

14. Lee SD, Kim SH, Kong SY, Kim YK, Park SJ. Kinetics of B, T, NK lymphocytes and isoagglutinin titers in ABO incompatible living donor liver transplantation using rituximab and basiliximab. Transpl Immunol. 2015; 32:29–34.

15. Kim JM, Kwon CH, Joh JW, Kang ES, Park JB, Lee JH, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013; 59:1215–1222.

16. Lee EC, Kim SH, Shim JR, Park SJ. A comparison of desensitization methods: rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2018; 17:119–125.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download