Abstract
Background
This study was designed to analyze the clinical usefulness of mycophenolic acid trough concentration monitoring in kidney transplantation patients who were maintained with cyclosporine.
Methods
The data of patients who underwent mycophenolic acid trough concentration monitoring after their first kidney transplant between November 2006 and August 2013 and were prescribed with cyclosporine, mycophenolate, and methylprednisolone were reviewed retrospectively. Cox analysis was used to analyze the risk factors for acute rejection within 1 year post-transplantation.
Results
Among 90 patients, 41 (45.6%) achieved both the target levels of cyclosporine and mycophenolic acid, while three patients (3.3%) failed to achieve the target level of either cyclosporine or mycophenolic acid. Nine patients (10.0%) only achieved the mycophenolic acid target level and 37 patients (41.1%) only achieved the cyclosporine target level. While patients who achieved only the mycophenolic acid target concentration had no statistically increased risk compared to patients who achieved both target levels (hazard ratio [HR], 1.569; 95% confidence interval [CI], 0.316 to 7.778; P=0.581), patients who only achieved the cyclosporine target concentration showed an increased risk of rejection compared to the both achievement group (HR, 4.112; 95% CI, 1.583 to 10.683; P=0.004). Patients who had no achievement in the target levels showed significantly increased rejection risk compared to the patients who achieved both target levels (HR, 17.811; 95% CI, 3.072 to 103.28; P=0.001).
Mycophenolate mofetil (MMF) and enteric-coated mycophenolate sodium (EC-MPS) is one of the key immunosuppressive agent indicated for the prevention of acute rejection after kidney transplantation (KT)(1). Currently, multiple agents are used for optimal immunosuppression with lower toxicities in KT(2). Calcineurin inhibitors (CNIs; tacrolimus and cyclosporine), mycophenolate (MMF and EC-MPS), and corticosteroid are considered to be the key components of the triple immunosuppressive regimen for maintenance(2).
The wide inter- and intra-patient variability of mycophenolic acid (MPA) was an obstacle in utilizing mycophenolate in real practice(3). Although mycophenolate was better than azathioprine or placebo in combination with cyclosporine A (CsA) in preventing rejection(45), randomized studies that examined the benefit of therapeutic drug monitoring of MPA showed conflicting results(6).
Nevertheless, there is a consensus that drug monitoring is required for optimal patient management(7). MPA AUC0-12 (area under the concentration-time curve) is considered as a gold standard, and is significantly associated with clinical events(78910). However, technical impracticality has been a barrier to its acceptance in clinical practice. Limited sampling strategy is an option that can be recommended for practitioners instead of MPA AUC0-12(1112). However, single concentration such as the trough concentration has been resulted in an inconsistent finding throughout studies(61013141516).
Although tacrolimus is preferred compared to CsA in many centers around the world, CsA still has an important role in KT(1718). Our center started the therapeutic drug monitoring strategies on plasma MPA trough concentration since 2006 along with CsA and tacrolimus. As our center transplanted more than 2,000 kidneys with 10 years of experience in MPA trough concentration monitoring, we designed this study to investigate the clinical implication of monitoring MPA trough concentration in CsA using patients.
Patients who underwent MPA trough concentration monitoring after their first KT at Samsung Medical Center, Seoul, Korea, between November 2006 and August 2013 were retrospectively reviewed. Patients considered to be low risk for acute rejection who received the triple maintenance regimen of CsA, mycophenolate (either MMF or EC-MPS), and methylprednisolone were included to the study. By the definition of low-risk, the patients should not have a history of previous transplantation, positive results on complement-dependent cytotoxicity, flow cytometry, donor-specific antibody with a mean fluorescence intensity above 2,500 and ABO incompatibility with the donor. Exclusion criteria were patients who were considered to be high risk of acute rejection and are as follows: patients who underwent desensitization prior to KT; patients who underwent induction therapy with daclizumab, alemtuzumab, rituximab, or more than 3 days of thymoglobulin; previous history of KT; multiorgan transplantation; ABO incompatible transplantation; positive for donor-specific antibody; pediatric patients; cessation of main regimen within 1 month after KT; and other factors related to high risk KT. Patients' demographic information, immunosuppressive features, and clinical outcomes are summarized in Table 1. We included 90 patients, including 50 males (55.6%) and 40 females (44.4%). The mean recipient age and donor age were 42.54±11.35 and 42.07±11.45 years, respectively. Human leukocyte antigen (HLA) was matched in 10 patients (11.1%), and the mean number of HLA mismatches was 3.01±1.62. The mean donor serum creatinine level was 76.02 µmol/L (interquartile range [IQR], 24.75). Forty-seven transplants (52.2%) were a living-related, while 26 transplants (28.8%) were living-unrelated cases. Seventeen cases (19.0%) were cadaveric transplantation. During the study period, our center started to adopt a policy to perform protocol kidney biopsy at 12th post-KT day. The protocol was to perform ultrasonography-guided gun biopsy of the transplanted kidney unless there is a contraindication to perform biopsy, such as use of anticoagulant that can risk bleeding.
Patients received CsA, mycophenolate (either MMF or EC-MPS), and methylprednisolone as an initial triple immunosuppressive regimen. MMF was used in 29 patients (32.2%), while EC-MPS was used in 60 patients (66.7%). One patient switched from MMF to EC-MPS. Basiliximab (n=76, 84.4%) or 3 days of thymoglobulin (n=6, 6.7%) were used as an induction therapy. The dosage of CsA and mycophenolate were adjusted to maintain their target trough concentrations. The target level of CsA trough concentrations were 200 to 250 ng/mL during the first week after transplantation, 150 to 200 ng/mL during 1 week to 1 month post-transplantation and above 100 ng/mL afterwards. The target MPA trough concentration was 1.5 to 2.5 mg/L during the first 1 year after transplantation (1119). Methylprednisolone was tapered if the patient became stabilized.
MPA trough concentration was monitored on a routine manner along with CsA trough concentration. Both CsA trough concentration and MPA trough concentration were monitored by high-performance liquid chromatography with tandem mass spectrometry. During admission, laboratory test for MPA was performed several times, but not every day for dose adjustment. When patients visited the outpatient clinic, MPA trough concentration was measured along with other laboratory tests.
Patients' gender, age at KT, and the calculated body mass index (BMI) of both donor and recipient, mode and duration of renal replacement therapy, cause of renal failure, HLA status, serum creatinine of donor, relationship of the donor and recipient, cause of death of cadaveric donor, panel reactive antibody status, and CMV status were collected. Acute cellular rejection was diagnosed based on Banff criteria. Acute rejection which was diagnosed borderline was also included as rejection episode. Data of episodes of gastrointestinal complications (diarrhea or gastritis), cytopenia (anemia, neutropenia, or thrombocytopenia), infections (BK polyomavirus, cytomegalovirus, pneumonia, urinary tract infection, influenza, invasive fungal infection, Pneumocystis jiroveci infection, tuberculosis, and other infections), graft failures, and deaths were collected. BK polyomavirus infection was defined as detection of the virus in urine sample while cytomegalovirus infection was defined as positive antigenemia in blood samples with or without symptoms related to infection.
As the target level of CsA trough concentration changes with time after KT, mean CsA trough concentrations were calculated separately for within 1 month post-transplantation and 1 month to 1 year post-transplantation. Patient's target CsA trough concentration was categorized as ‘achieved,’ if both mean concentrations within 1 month post-transplantation and 1 month to 1 year were above 150 ng/mL and above 100 ng/mL, respectively. Patient's target MPA trough concentration was categorized as ‘achieved,’ if mean MPA trough concentration was above 1.5 mg/L during 1 year post-transplantation. Patients were categorized into four groups based on their achievements in target trough concentrations; achievements of both CsA and MPA, achievement of only MPA, achievement of only CsA, and neither achievement of CsA nor MPA. The mean CsA trough concentration and MPA trough concentration were calculated using Excel (Microsoft, Redmond, WA, USA).
Student's t-test was used to analyze the difference in mean CsA trough concentration and mean MPA trough concentration between patients who achieved only one target concentration and patients with both achievements. To analyze the risk factors for biopsy-proven acute rejection (BPAR), multivariable Cox proportional hazard ratio was used to analyze factors related to BPAR within 1 year post-transplantation. BPAR was set as an end point. Cessation of CsA, cessation of mycophenolate for longer than 3 months, and cessation of MPA monitoring were censored in the analysis. For the analysis, continuous variables were changed to categorical variables by dividing them into two groups. Recipient age and donor age were divided by 50 years and 40 years of age, respectively. Both recipient and donor BMI were classified as greater than or less than 25 kg/m2. HLA status was classified as matched or mismatched. Dialysis duration was divided by 90 days. P-values <0.05 were used to indicate statistical significance. Statistical analyses were performed with SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). This study was approved by the Institutional Review Board of Samsung Medical Center and was waived for the need of informed consent since it was designed as a retrospective study (IRB No. 2016-12-056).
BPAR occurred in 25 patients (27.8%) within 1 year post-transplantation. Since our center started protocol kidney biopsy starting August 2012, only eight patients (8.9%) underwent protocol kidney biopsy at their second week post-transplantation. Among these patients, only one patient (1.1%) was diagnosed BPAR, and two patients (2.2%) were diagnosed BPAR at their third month post-transplantation. Diarrhea and gastritis occurred in five patients (5.6%) and nine patients (10.0%), respectively. Neutropenia occurred in seven patients (7.8%). The most common infections were cytomegalovirus (n=56, 62.2%) and BK polyomavirus (n=37, 41.1%). Although graft failure occurred in three patients (3.3%), and death occurred in one patient (1.1%), the three graft failure cases (40, 70, and 53 months after KT) and expired case (56 months after KT) occurred after the period of 1 year post-transplantation.
Table 2 shows the mean trough concentrations categorized by target trough concentration achievements. The mean CsA trough levels were 217.2±48.9 and 172.3±47.7 ng/mL during the follow up periods of 1 month and 1 month to 1 year post-KT, respectively. Mean 1 month and 1 month to 1 year CsA trough concentrations were 131.2±14.8 and 193.9±67.0 ng/mL in no achievement group; 238.8±40.4 and 184.8±48.4 ng/mL in CsA only achievement group; 136.7±19.4 and 133.3±45.5 ng/mL in MPA only achievement group; and 225.4±37.4 and 169.0±43.7 ng/mL in both achievements group, respectively.
Median 1 month MPA trough concentration and mean 1 month to 1 year concentration were 1.36 (IQR, 1.89) and 2.16±1.50, respectively. Median 1 month and mean 1 month to 1 year MPA trough concentrations were 0.85 and 1.10±0.86 mg/L in no achievement group; 0.80 (IQR, 0.88) and 0.96±0.39 mg/L in CsA only achievement group; 2.60 (IQR, 3.49) and 3.31±1.83 mg/L in MPA only achievement group; and 1.89 (IQR, 1.86) and 2.95±1.35 mg/L in both achievements group, respectively.
Multivariable Cox analysis was performed including the significant factors related to BPAR-free survival in the univariable analyses. (Table 3) Donor age ≥40 years was significantly related to BPAR-free survival (hazard ratio [HR], 2.265; 95% confidence interval [CI], 1.014 to 5.059; P=0.046) in the univariable analysis. While recipient age ≥ 50 years showed a trend of better BPAR-free survival compared to <50 years, it was statistically insignificant (HR, 0.362; CI, 0.124 to 1.056; P=0.063). CsA target was achieved in 78 patients (86.7%) while 12 patients (13.3%) failed to achieve the target concentration. MPA target was achieved in 50 patients (55.6%) while 40 patients (44.4%) failed to achieve the target concentration. When patients were divided into four groups based on their achievements in mean target trough concentrations, nine patients (10.0%) achieved only MPA concentration, 37 patients (41.1%) achieved only CsA concentration, and three patients (3.3%) had no achievement in both target levels. The four patient groups showed significant difference in their BPAR-free survival (P=0.023) according to univariable Cox analysis.
Multivariable Cox analysis including donor age and target level achievements showed that donor age ≥40 years (HR, 3.573; CI, 1.307 to 9.766; P=0.013) and target level achievements (P=0.003) were both significant factors for BPAR-free survival. While patients who achieved only MPA target concentration had no statistically increased risk compared to patients who achieved in both target levels (HR, 1.569; CI, 0.316 to 7.778; P=0.581), patients who only achieved CsA target concentration showed increased risk of BPAR compared to both achievement group (HR, 4.112; CI, 1.583 to 10.683; P=0.004). Patients who had no achievement in target levels showed significantly increased BPAR risk compared to patients who achieved in both target levels (HR, 17.811; CI, 3.072 to 103.28; P=0.001).
Fig. 1 shows the survival curves of the three patient groups categorized by their achievements in mean CsA and MPA trough concentrations.
Analyses on the toxic effect of mycophenolate, namely infections, gastrointestinal toxicities and cytopenia were also performed. Patients were divided into two groups based on MPA trough concentration of 2.5 mg/L and analyzed with the risk of any infections, gastrointestinal disturbance and cytopenia. Kaplan-Meier log rank test showed that there were no differences between the two groups regarding infection (P=0.546), gastrointestinal disturbance (P=0.055), and cytopenia (P=0.601).
Although previously published studies showed conflicting results of MPA trough concentration monitoring(202122), our center started monitoring MPA trough concentrations since November 2006 as a routine procedure. Although our center did not use CsA as the main CNI during the period, we have accumulated a reasonable amount of data on MPA trough monitoring in CsA using patients. We previously published our study on the clinical usefulness of monitoring MPA trough concentration in tacrolimus users(23). However, CsA is still used in many patients especially when tacrolimus cannot be tolerated or patients' preference regarding the side effects. That is why we decided to perform this study for seeking the clinical implication of MPA monitoring. Our study focused on acute cellular rejection within 1 year post-transplantation and analyzed the clinical implication of mean MPA trough concentration along with mean CsA trough concentration and other factors. While previous retrospective studies on MPA trough concentration were analyzed without controlling the confounding factors, we tried to adjust the effect of other potential co-variables.
Target achievement in mean CsA trough concentration was defined as mean CsA trough concentration above 150 ng/mL during the first month post-transplantation and above 100 ng/mL during 1 month to 1 year post-transplantation, respectively. On the other hand, achievement in MPA trough concentration was determined when a patient had mean MPA trough concentration above 1.5 mg/L throughout the period of 1 year post-transplantation. Patients were categorized into four groups based on the achievements in target concentration of CsA and MPA. The result shows that patients who achieved both CsA and MPA target concentration had the lowest risk of BPAR while, the risk increased with unachieved target concentrations. Based on the different calculated risk of MPA only achieved patients and CsA only achieved patients, it is assumable that the achievement of MPA trough concentration was more influential compared to CsA achievement. However, the fact that only 12 patients failed to achieve CsA target level while 40 patients failed to achieve MPA target should be noticed. Most of the patients reached their target level of CsA trough concentration and this makes it difficult to reveal the true importance of CsA on BPAR.
Multivariable analysis of risk factors for BPAR-free survival showed that patients' risk for BPAR increases as they failed to achieve the target trough concentration (Table 3, Fig. 1). Mycophenolate (P=0.165), whether MMF or EC-MPS was used, was not a significant factor for BPAR-free survival. Whether the patient underwent protocol kidney biopsy was also not a significant factor (P=0.543).
In our center, we monitor the patients with their MPA trough concentration and use it as a parameter of dose adjustment. However, due to the intra-patient variability of MPA trough concentration, we do not change the dosage of mycophenolate as abundantly as CNIs. Usually, EC-MPS of 540 mg twice a day and MMF of 750 mg twice a day is prescribed initially with some adjustment according to their bodyweight. Mycophenolate dosage is adjusted only when there is a consecutive laboratory results with underor over-dosage.
The limitation of this study comes from the inborn nature of a retrospective observational study. The selection of 90 patients out of more than 2,000 kidney-transplanted patients possesses a possibility of selection biases. Structured as a single institutional study with a homogenous ethnicity, this study should be cautiously interpreted, especially for KT patients with different ethnicity. Although this study was organized as a multivariable study, the factors significantly related to BPAR-free survival in the univariable analysis were only donor age, CsA and MPA. Other factors showed insignificant relationship with BPAR in the univariable analysis. This might be due to the low number of study subject. Because the target concentration becomes lower as follow up duration gets longer, finding a universal cut-off point of mean trough concentration was impossible. That is why this study focused on the first year after transplantation, when acute cellular rejection occurs predominantly, requiring precaution in patient management.
Another limitation that should be mentioned is that, this study defined target achievement of MPA as mean MPA trough concentration above 1.5 mg/L without any upper limit. This was because that the main goal was to analyze the relationship of mean MPA trough concentration and BPAR, which risk increases as the level gets lower. Therefore, we did not consider that above the appropriate therapeutic range does not have any impact on BPAR compared to the true target range. However, the fact that levels above 2.5 mg/L of MPA trough concentration were all considered to be achieving the target should be mentioned.
Although tacrolimus is preferred as a maintenance immunosuppressant in many transplantation centers around the world, CsA is still an important CNI, which can be used in patients who experience drug toxicities of tacrolimus. In patients with a CsA regimen, mycophenolate should be monitored because pharmacokinetic property can be influenced by CsA, resulting in different drug exposure with a same dosage compared to tacrolimus. Our study showed the importance of achieving a mean target concentration of both CsA and MPA during the first year after KT to reduce the risk of BPAR. Although the result of our study should be interpreted with caution to avoid the influence of potential selection biases mentioned above, we carefully suggest that MPA trough concentration monitoring can be safely utilized in the clinical setting of the management of KT patient on a CsA regimen.
In patients who are under triple maintenance immunosuppressive regimen of CsA, mycophenolate, and corticosteroids after their first KT, risk of BPAR within 1 year post-transplantation can be significantly reduced when the therapeutic target of both mean CsA trough concentration and mean MPA trough concentration are achieved.
Figures and Tables
Fig. 1
Biopsy-proven acute rejection-free survival curves of patients categorized by three groups: Patients who achieved both target levels of cyclosporine A (CsA) and mycophenolic acid (MPA) (n=41), patients who only achieved one target level (n=46), and patients who failed to achieve neither of the target levels (n=3). The hazard ratios (HRs) of the latter two subgroups were calculated comparing to the biopsy-proven acute rejection-free survival of the first subgroup. Abbreviation: CI, confidence interval.
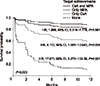
Table 1
Patient demographics, immunosuppressive regimens, and clinical outcome of low risk kidney transplantation patients who had triple maintenance regimen of cyclosporine, mycophenolate, and methylprednisolone

Table 2
Mean trough levels of cyclosporine and mycophenolic acid categorized by treatment period of 1 month and 1 month to 1 year and target mean trough concentration achievements

References
1. Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009; 87:785–794.

2. Bunnapradist S, Sampaio MS, Wilkinson AH, Pham PT, Huang E, Kuo HT, et al. Changes in the small bowel of symptomatic kidney transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium. Am J Nephrol. 2014; 40:184–190.

3. Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998; 34:429–455.

4. Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000; 47:215–245.

5. Wagner M, Earley AK, Webster AC, Schmid CH, Balk EM, Uhlig K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2015; 12:CD007746.

6. Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010; 5:341–358.

8. Hale MD, Nicholls AJ, Bullingham RE, Hene R, Hoitsma A, Squifflet JP, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998; 64:672–683.

9. van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999; 68:261–266.

10. Tett SE, Saint-Marcoux F, Staatz CE, Brunet M, Vinks AA, Miura M, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev (Orlando). 2011; 25:47–57.

11. van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006; 28:145–154.

12. Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tonshoff B. Validation of an abbreviated pharmacokinetic profile for the estimation of mycophenolic acid exposure in pediatric renal transplant recipients. Ther Drug Monit. 2006; 28:623–631.

13. Borrows R, Chusney G, Loucaidou M, James A, Lee J, Tromp JV, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006; 6:121–128.

14. Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, et al. Long-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected by trough plasma concentration: a prospective study in 100 de novo renal allograft recipients. J Clin Pharmacol. 2003; 43:866–880.

15. Le Meur Y, Buchler M, Thierry A, Caillard S, Villemain F, Lavaud S, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007; 7:2496–2503.

16. Weber LT, Shipkova M, Armstrong VW, Wagner N, Schutz E, Mehls O, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic Acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol. 2002; 13:759–768.

17. Gardiner KM, Tett SE, Staatz CE. Multinational evaluation of mycophenolic acid, tacrolimus, cyclosporin, sirolimus, and everolimus utilization. Ann Transplant. 2016; 21:1–11.

18. Axelrod DA, Naik AS, Schnitzler MA, Segev DL, Dharnidharka VR, Brennan DC, et al. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. Am J Transplant. 2016; 16:2453–2462.

19. Miura M, Niioka T, Kato S, Kagaya H, Saito M, Habuchi T, et al. Monitoring of mycophenolic acid predose concentrations in the maintenance phase more than one year after renal transplantation. Ther Drug Monit. 2011; 33:295–302.

20. Budde K, Bauer S, Hambach P, Hahn U, Roblitz H, Mai I, et al. Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant. 2007; 7:888–898.

21. Cattaneo D, Cortinovis M, Baldelli S, Bitto A, Gotti E, Remuzzi G, et al. Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients. Clin J Am Soc Nephrol. 2007; 2:1147–1155.

22. de Winter BC, van Gelder T, Glander P, Cattaneo D, Tedesco-Silva H, Neumann I, et al. Population pharmacokinetics of mycophenolic acid : a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet. 2008; 47:827–838.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download