Abstract
Antibody-mediated rejection (AMR) is a major complication after ABO-incompatible liver transplantation. According to the 2016 Banff Working Group on Liver Allograft Criteria for the diagnosis of acute AMR, a positive serum donor specific antibody (DSA) is needed. On the other hand, the clinical significance of the histological findings of AMR in the absence of DSA is unclear. This paper describes a 57-year-old man (blood type, O+) who suffered from hepatitis B virus cirrhosis with hepatocellular carcinoma. Pre-operative DSA and cross-matching were negative. After transplantation, despite the improvement of the liver function, acute AMR was observed in the protocol biopsy on postoperative day 7; the cluster of differentiation 19+ (CD19+) count was 0% and anti-ABO antibody titers were 1:2. This paper presents the allograft injury like AMR in the absence of DSA after ABOi living donor liver transplantation with low titers of anti-ABO antibody and depleted serum CD19+ B cells.
Go to : 
REFERENCES
1). Cooper DK. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent). 2012. 25:49–57.

2). Gugenheim J., Samuel D., Reynes M., Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990. 336:519–23.

3). Usuda M., Fujimori K., Koyamada N., Fukumori T., Sekiguchi S., Kawagishi N, et al. Successful use of anti-CD20 mono-clonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005. 79:12–6.

4). Egawa H., Teramukai S., Haga H., Tanabe M., Fukushima M., Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008. 47:143–52.

5). Demetris AJ., Bellamy C., Hubscher SG., O'Leary J., Randhawa PS., Feng S, et al. 2016 Comprehensive update of the Banff Working Group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016. 16:2816–35.
6). Krishnan NS., Zehnder D., Briggs D., Higgins R. Human leukocyte antigen antibody incompatible renal transplantation. Indian J Nephrol. 2012. 22:409–14.

7). Song GW., Lee SG., Hwang S., Kim KH., Ahn CS., Moon DB, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016. 16:157–70.

8). Castillo-Rama M., Castro MJ., Bernardo I., Meneu-Diaz JC., Elola-Olaso AM., Calleja-Antolin SM, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 2008. 14:554–62.

9). Kozlowski T., Rubinas T., Nickeleit V., Woosley J., Schmitz J., Collins D, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011. 17:357–68.

10). O'Leary JG., Kaneku H., Jennings LW., Banuelos N., Susskind BM., Terasaki PI, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013. 19:973–80.
11). Taner T., Gandhi MJ., Sanderson SO., Poterucha CR., De Goey Sr., Stegall MD, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012. 12:1504–10.

12). Kaneku H., O'Leary JG., Banuelos N., Jennings LW., Susskind BM., Klintmalm GB, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013. 13:1541–8.
13). Zou Y., Heinemann FM., Grosse-Wilde H., Sireci G., Wang Z., Lavingia B, et al. Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum Immunol. 2006. 67:230–7.

14). Alten TA., Negm AA., Voigtlander T., Jaeckel E., Lehner F., Brauner C, et al. Safety and performance of liver biopsies in liver transplant recipients. Clin Transplant. 2014. 28:585–9.

15). O'Leary JG., Kaneku H., Demetris AJ., Marr JD., Shiller SM., Susskind BM, et al. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014. 20:218–27.
Go to : 
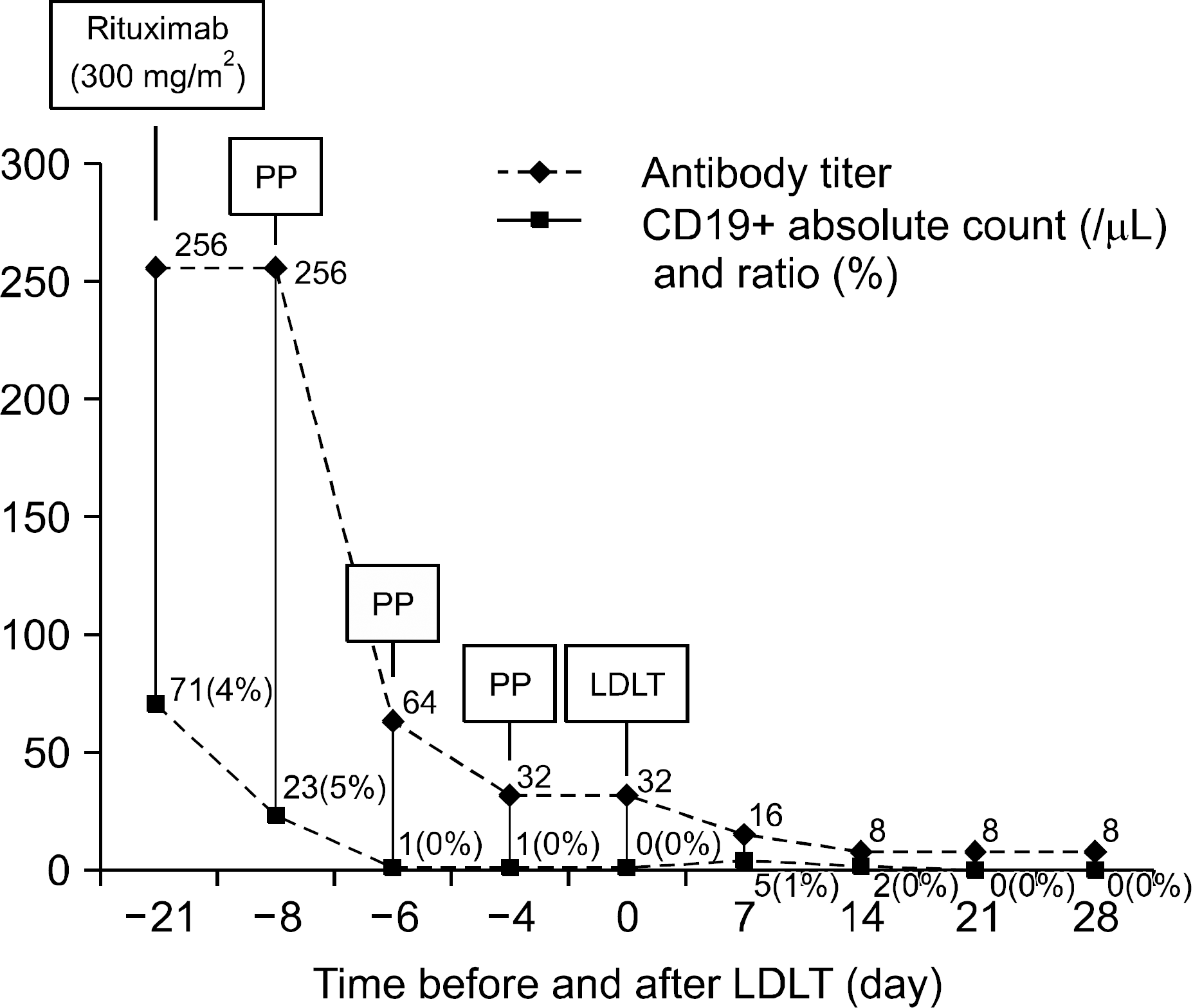 | Fig. 1.Desensitization protocol and changes in the cluster of differentiation 19+ (CD19+) lymphocyte count and the titer of anti ABO antibody. The titers of anti-ABO antibodies decreased to 1:8 from 1:256, and serum CD19+ B cells were depleted before the operation. Abbreviations: PP, plasmapheresis; LDLT, living donor liver transplantation. |
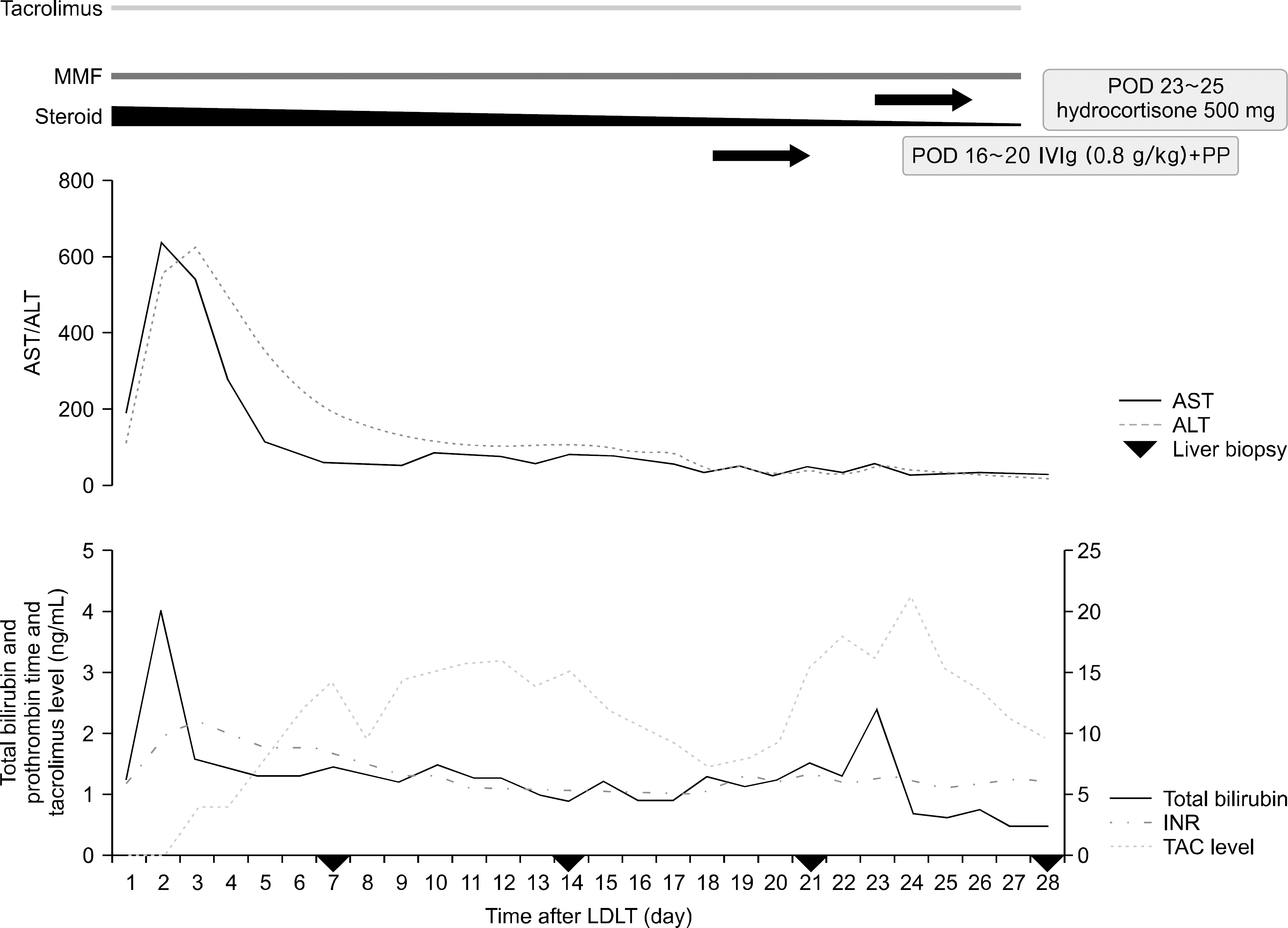 | Fig. 2.Changes in aspartate aminotransferase (AST)/alanine aminotransferase (ALT), total bilirubin, prothrombin time (INR), and tacrolimus (TAC) level after living donor liver transplantation (LDLT). We performed liver biopsy on postoperative day (POD) 7, 14, 21, and 28. After pathologic confirm the acute antibody-mediated rejection, we conducted rejection therapy (POD 16 to 20, intravenous immunoglobulin [IVIg, 0.8 g/kg] for 3 days and plasmapheresis [PP]; POD 23 to 25, steroid pulse therapy [500 mg of hydrocortisone 500 mg for 3 days then tapering]). Abbreviation: MMF, myophenoate mofetil. |
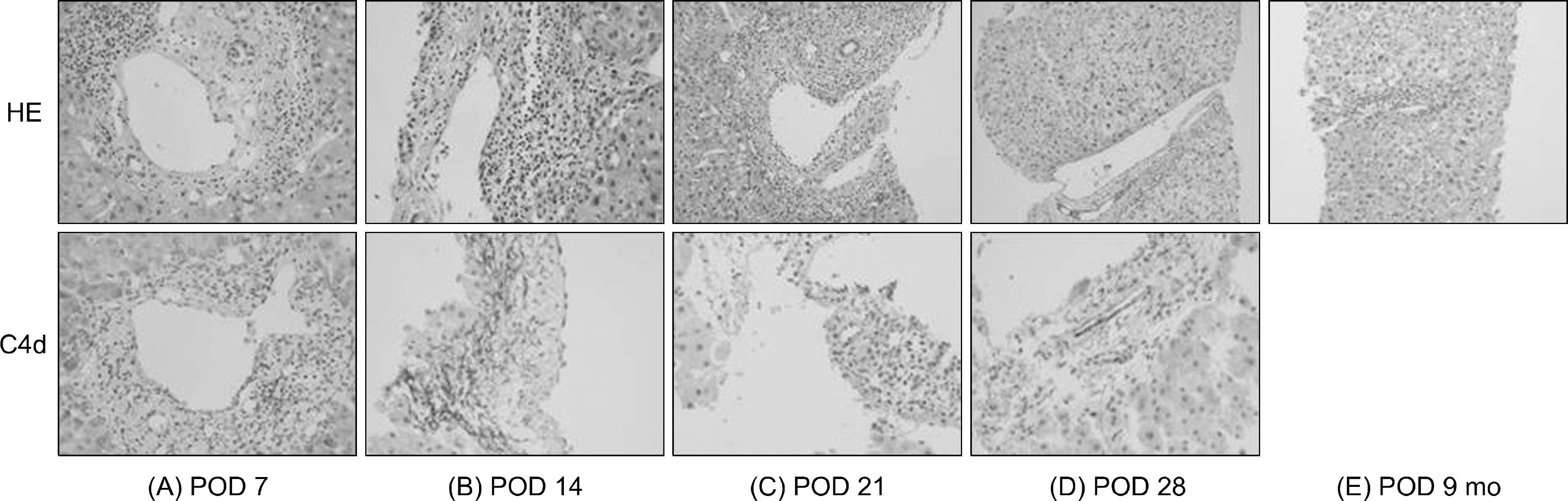 | Fig. 3.Liver histology: (A) postoperative day (POD) 7 (×400), (B) POD 14 (×400), (C) POD 21 (×400), (D) POD 28 (×400), (E) POD 9 months (×400) (HE stain and C4d deposition). (A) POD 7 (2016 Banff Criteria H-score 1, C4d score 2): mild portal infiltration of neutrophils and some eosinophils with the presence of portal endothelial and stromal complement component 4d (C4d) deposition, The pathologist reported that the possibility of acute antibody-mediated rejection (AMR) in ABO-incompatible grafts could not be excluded. (B) POD 14 (2016 Banff Criteria H-score 1, C4d score 2): portal inflammation involving most of the portal tracts with portal venous endotheliitis with endothelial cell hypertrophy and mixed lymphocytes, neutrophils and eosiophils were as observed on postoperative day 14. Moreover, the immunofluorescence staining showed a linear pattern of C4d staining on the endothelial cell and stroma. Additional donor specific antibody results after transplantation were also negative. (C) POD 21 (2016 Banff Criteria H-score 2, C4d score 2): acute AMR findings were still observed on pathologic examination and aggravated degree of portal inflammation and endotheliitis. (D) POD 28 (2016 Banff Criteria H-score 1, C4d score 0): histological analysis on postoperative day 28 showed that inflammatory cell infiltration was localized in one portal tract and that hepatocellular swelling, a change induced by steroid pulse therapy, was observed in liver allografts. (E) POD 9 months: the histological features showed that no inflammatory changes in the portal tract and the absence of portal endothelial and stromal C4d deposition. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download