Abstract
Background
Recently, we examined the effects of 2% lidocaine gel on the tactile sensory and pain thresholds of the face, tongue and hands of symptom-free individuals using quantitative sensory testing (QST); its effect was less on the skin of the face and hands than on the tongue. Consequently, instead of 2% lidocaine gel, we examined the effect of 8% lidocaine spray on the tactile sensory and pain thresholds of the skin of the face and hands of healthy volunteers.
Methods
Using Semmes-Weinstein monofilaments, QST of the skin of the cheek and palm (thenar skin) was performed in 20 healthy volunteers. In each participant, two topical sprays were applied. On one side, 0.2 mL of 8% lidocaine pump spray was applied, and on the other side, 0.2 mL of saline pump spray was applied as control. In each participant, QST was performed before and 15 min after each application. Pain intensity was measured using a numeric rating scale (NRS).
Results
Both the tactile detection threshold and filament-prick pain detection threshold of the cheek and thenar skin increased significantly after lidocaine application. A significant difference between the effect of lidocaine and saline applications was found on the filament-prick pain detection threshold only. NRS of the cheek skin and thenar skin decreased after application of lidocaine, and not after application of saline.
Temporomandibular disorders (TMD), trigeminal neuralgia (TN) and burning mouth syndrome (BMS) are painful orofacial conditions that dentists must assess, diagnose, and manage [12345].
First-line therapy for TN includes carbamazepine and oxcarbazepine [4]. On the other hand, medications regularly used for other neuropathic pains have been used for BMS [5]. There are expert opinions that topical lidocaine may be useful in the management of these orofacial pain conditions [45]. In an earlier study, Tamakawa and Ogawa [6] applied 60% lidocaine tape (Penles), which is used to anesthetize skin when an intravenous catheter is inserted, for the management of post-herpetic neuralgia (PHN). They reported that lidocaine tape induced minor side-effects, erythema in one patient and increased pain in another patient [6]. Niki et al. [7] applied 8% lidocaine spray to the oral mucosa in patients with TN. Kanai et al. [8] also applied it in patients with PHN. Based on the prompt analgesia, lack of systemic side effects, and convenience of use, they recommended 8% lidocaine spray for the management of TN and PHN [78].
Recently, we examined the effects of 2% lidocaine gel on the tactile sensory and pain thresholds of the face, tongue, and hands of symptom-free individuals using quantitative sensory testing (QST) [9]. Our data indicated that sensory and pain thresholds increased after 2% lidocaine gel application, but as compared to the tongue, the effects on the skin of the face and hands were not significant [9].
Consequently, in the present study we examined the effect of 8% lidocaine (LDC) spray, instead of 2% lidocaine gel, on the tactile sensory and pain thresholds of the skin of the face and hands of healthy volunteers.
Prior to the experiment, we estimated the appropriate sample size using statistical tools (JMP Pro14), to determine the significant difference in pain thresholds estimated using Semmes-Weinstein monofilaments (Premier Products, Kent, WA, USA). The mean and standard deviation (SD) values for pain thresholds with topical lidocaine were 2.10 ± 0.63 (Log Force) in our previous study [9]. Based on the performance and characteristics of repeated measurements of pain thresholds, we estimated that a sample size of twenty participants would have 80% power to detect a difference in pain thresholds of 0.3 to 0.5 (Log Force).
Twenty healthy volunteers participated. Informed consent was obtained from all participants. The Ethics Committee of Nagasaki University Graduate School of Biomedical Sciences approved the study (No. 1502), which adhered to the guidelines of the Helsinki Declaration.
In each participant, QST was performed on the cheek skin (CS) and palm of the hand (thenar skin, TS) before and 15 min after each application. Two types of topical application were used in each participant. On one side, 0.2 mL of LDC pump spray (Xylocaine pump spray, Astra Zeneka) was applied, and on the other side, 0.2 mL of saline (SAL) pump spray was applied as control. The sides (left and right) were randomised.
Tactile detection threshold (TDT) and filament-prick pain detection threshold (FPT) were determined using Semmes-Weinstein monofilaments. The instrument and procedure have been described in detail elsewhere [91011121314151617].
The participants were instructed to close their eyes during the TDT procedure. The TDT was measured using the stair-case method [91011121314151617].
After the TDT measurements, the FPT was examined in the same manner. However, the participants were instructed to keep their eyes open throughout the FPT procedure. The interval between measurements at the same site was set at 3 min in order to avoid sensitization. Pain intensity of the FPT was also assessed on a numeric rating scale (NRS) [91011121314151617].
The mean values and standard error (SEM) of TDT and FPT were calculated. Since data were not normally distributed, Wilcoxon-matched pair test was performed to test the effects of LDC and/or SAL. Wilcoxon-Mann-Whitney test was used to compare the gender (men and women) and side (left and right) differences. A significant difference was accepted at P < 0.05.
The participants comprised 10 men and 10 women. The mean age of the male participants was 28.3 ± 6.1 years (age range from 23 to 42 years), and that of the female paticipants was 24.5 ± 1.0 years (age range from 23 to 26 years). No participant withdrew from this experiment.
Before application of LDC and/or SAL, we compared the pre-TDT, FPT, and NRS between the right and left sides. There were no significant differences between the sides (Table 1). In addition, there were no significant gender differences in any of the parameters. Consequently, all data were averaged in order to obtain a single value (Table 2).
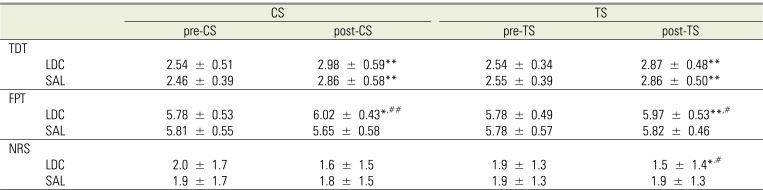
The TDT significantly increased after application, regardless of LDC/SAL (CS: P < 0.01, TS: P < 0.01), and there were no significant differences between LDC and SAL at any site (CS and TS) (Table 2).
The FPT significantly increased after application of LDC (CS: P < 0.05, TS: P < 0.01). After application of SAL, the FPT at the TS remained stable but that at the CS decreased from 5.81 ± 0.55 to 5.65 ± 0.58. There were significant differences between LDC and SAL application at both sites (CS: P < 0.01, TS: P < 0.05) (Table 2).
There were no differences in the NRS between before and after application of SAL. On the other hand, the NRS decreased after application of LDC from 2.0 ± 1.7 to 1.6 ± 1.5 at the CS, and from 1.9 ± 1.3 to 1.5 ± 1.4 at the TS (P < 0.05). At the TS, there was a significant difference between LDC and SAL application (P < 0.05) (Table 2).
Our results showed that both TDT and FPT on the CS and TS increased significantly after LDC application, but significant differences between LDC and SAL applications was found on the FPT only, illustrating the effect of LDC application. In addition, we could clarify that the effect of LDC spray was more pronounced than that of 2% lidocaine gel by comparing this result to that of our previous study [9].
Carbamazepine and oxcarbazepine have been used in clinical practice as first-line therapy for the management of TN [4]. These drugs remain the gold standard, but they also have side effects such as sleepiness, staggery, nausea, vomiting and drug eruptions [4]. Recently, topical lidocaine has been suggested and accepted as complementary or alternative medicine for TN [4]. In fact, Niki et al. [7] and Kanai et al. [8] showed the effect of 8% lidocaine spray in the treatment of neuropathic pain such as TN and PHN, respectively, without serious side effects. Sakai et al. [18] applied 10% lidocaine gel to the forearms of healthy volunteers, and quantitatively evaluated the effect of transdermal lidocaine on differential sensory nerve block using current perception threshold testing.
The fact that the pain threshold is more susceptible than the sensory threshold to local anesthetics can be explained by the “size principle”, i.e. the sensitivity of fibers to local anesthetics is inversely proportional to the axon diameter [18]. A-delta and unmyelinated C fibers are thinner than A-beta fiber [1920]. The former, related to pain, are more affected by lidocaine than the latter, which is involved in touch and pressure stimuli [1920]. In addition to nerve fibers, receptors are also involved in sensory perception [141920]. Sakai et al. [18] explained that transdermal lidocaine could affect the cold and pinprick receptors more strongly than the touch and warmth receptors, and that the intensity of blockade was stronger at the site of receptors than at the site of fibers. There are both free nerve endings and a variety of specialized nerve endings. Free nerve endings are associated with pain and temperature, and specialized nerve endings are involved in touch and pressure [19]. The differences in the terminals and/or receptors as well as the fibers should be taken into accout when considering the different reactions between the TDT and FPT to local anesthetics.
The increase of TDT after lidocaine application is habituation, because the same phenomenon was also found after saline application. Habituation is a decrease or loss of response following repetitive stimulation [913]. The opposite of habituation is sensitization, i.e. the increased excitability of a reaction to repetitive stimulation [913]. The decrease in FPT after saline application at the CS is the result of sensitization, but it was not found at the TS. Presence and absence of sensitization at the CS and TS were also found in our previous study [9]. As mentioned above, there are some morphological differences in the receptors between the skin of the face and hands [1419]. In additon, we suppose the presence of sensitization is related to the absence of visual perception, i.e. the sensitivity to repetitive stimulation on the face could be enhanced more than on the hand as compensatory reaction for lack of visual perception.
Clinically, a difference of at least 3 points in the NRS score is considered significant [78]. Niki et al. [7] and Kanai et al. [8] reported that LDC significantly reduced the NRS score from 5 to 1 in patients with TN, and from 6 to 1 in patients with PHN, respectively. In this study, the paticipants were all healthy volunteers, and their NRS scores before application were all under 3 points. Considering this, the NRS itself and its decrease after LDC were found to be small in this study, but could be valuable when comparing findings in patients with neuropathic pain. Topical lidocaine can be effective in patients with neuropathic pain [78], especailly if peripheral factors are more involved in the pathophysiology. In other words, QST can help to assess whether the pathogenesis of neuropathic pain is more associated with peripheral than central factors [20]. Consequently, as a next step, a case-control study is needed to clarify the effect of topical lidocaine in patients with neuropathic pain.
In conclusion, the effect of LDC spray on the sensory and pain thresholds of the skin of the face and hands was evaluated objectively using QST (measuring TDT and FPT) and NRS scores.
References
1. Honda M, Iida T, Komiyama O, Masuda M, Uchida T, Nishimura H, et al. Characteristics of middle-aged and older patients with temporomandibular disorders and burning mouth syndrome. J Oral Sci. 2015; 57:355–360. PMID: 26666859.

2. De Laat A. Pain associated with temporomandibular disorders and with burning mouth syndrome. In : Mogil J, editor. Pain 2010-an updated review: refresher course syllabus. Seattle: IASP press;2010. p. 147–152.
3. Komiyama O, Obara R, Uchida T, Nishimura H, Iida T, Okubo M, et al. Pain intensity and psychosocial characteristics of patients with burning mouth syndrome and trigeminal neuralgia. J Oral Sci. 2012; 54:321–327. PMID: 23221157.

4. Lewis MA, Sankar V, De Laat A, Benoliel R. Management of neuropathic orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103(Suppl 1):S32.e1–S32.e24. PMID: 17379152.

5. Patton LL, Siegel MA, Benoliel R, De Laat A. Management of burning mouth syndrome: systematic review and management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103(Suppl 1):S39.e1–S39.e13. PMID: 17379153.

6. Tamakawa S, Ogawa H. Lidocaine tape (Penles--a dressing tape based on 60% lidocaine--) reduces the pain of postherpetic neuralgia. Masui. 1998; 47:882–884. PMID: 9720343.
7. Niki Y, Kanai A, Hishi K, Okamoto H. Immediate analgesic effect of 8% lidocaine applied to the oral mucosa in patients with trigeminal neuralgia. Pain Med. 2014; 15:826–831. PMID: 24506194.

8. Kanai A, Kumaki C, Niki Y, Suzuki A, Tazawa T, Okamoto H. Efficacy of a metered-dose 8% lidocaine pump spray for patients with post-herpetic neuralgia. Pain Med. 2009; 10:902–909. PMID: 19682274.

9. Okayasu I, Komiyama O, Ayuse T, De Laat A. Effects of topical lidocaine in the oral and facial regions on tactile and sensory thresholds. Arch Oral Biol. 2016; 72:51–55. PMID: 27541635.
10. Okayasu I, Oi K, De Laat A. The effect of tooth clenching on the sensory and pain perception in the oro-facial region of symptom-free men and women. J Oral Rehabil. 2009; 36:476–482. PMID: 19486270.

11. Okayasu I, Oi K, De Laat A. The effect of nonfunctional tooth contact on sensory and pain perception in patient with myofascial pain of the jaw muscles. J Prosthodont Res. 2012; 56:87–92. PMID: 22424869.
12. Okayasu I, Komiyama O, Yoshida N, Oi K, De Laat A. Effects of chewing efforts on sensory and pain thresholds in human facial skin: A pilot study. Arch Oral Biol. 2012; 57:1251–1255. PMID: 22445779.
13. Okayasu I, Komiyama O, Ayuse T, De Laat A. Tactile sensory and pain thresholds in the face and tongue of subjects asymptomatic for oro-facial pain and headache. J Oral Rehabil. 2014; 41:875–880. PMID: 25041286.

14. Jacobs R, Wu C-H, van Loven K, Desnyder M, Kolenaar B, van Steenberghe D. Methodology of oral sensory tests. J Oral Rehabil. 2002; 29:720–730. PMID: 12220338.

15. Komiyama O, De Laat A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain. 2005; 115:308–315. PMID: 15911157.

16. Komiyama O, Gracely RH, Kawara M, De Laat A. Intraoral measurement of tactile and filament-prick pain threshold using shortened Semmes-Weinstein monofilaments. Clin J Pain. 2008; 24:16–21. PMID: 18180631.

17. Svensson P, Baad-Hansen L, Pigg M, List T, Eliav E, Ettlin D, et al. Guidelines and recommendations for assessment of somatosensory function in oro-facial pain conditions- a taskforce report. J Oral Rehabil. 2011; 38:366–394. PMID: 21241350.
18. Sakai T, Tomiyasu S, Yamada H, Ono T, Sumikawa K. Quantitative and selective evaluation of differential sensory nerve block after transdermal lidocaine. Anesth Analg. 2004; 98:248–251. PMID: 14693629.

19. Sessle BJ. Mechanisms of oral somatosensory and motor functions and their clinical correlates. J Oral Rehabil. 2006; 33:243–261. PMID: 16629880.

20. Eliav E, Gracely RH, Nahlieli O, Benoliel R. Quantitative sensory testing in trigeminal nerve damage assessment. J Orofac Pain. 2004; 18:339–344. PMID: 15636018.
Table 1
Tactile sensory and pain thresholds before application of lidocaine and/or saline
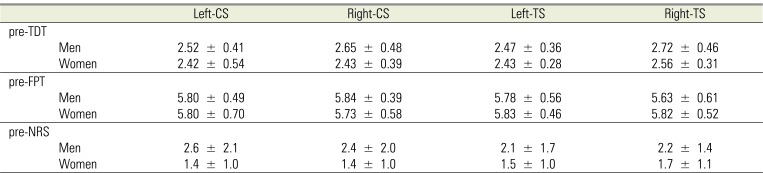
Table 2
Tactile sensory and pain thresholds before (pre) and after (post) application of lidocaine and/or saline




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download